Animated PowerPoint
advertisement

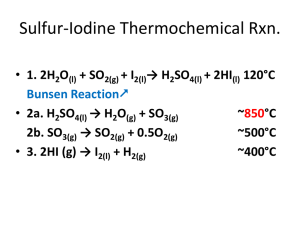
Lecture 25 Chemical Reaction Engineering (CRE) is the field that studies the rates and mechanisms of chemical reactions and the design of the reactors in which they take place. Web Lecture 25 Class Lecture 21– 4/2/2013 CSI • Ammonium Nitrate Explosion • Monsanto Explosion • T2 Laboratories Explosion 2 Case 1 – Ammonium Nitrate Explosion Massive blast at Terra plant kills four. 3 Example 1: Safety in Chemical Reactors H 2 O Gas N O 2 T0 200 F m A0 310 lb h 17 % H 2 O P 200°F Liquid 83 % NH 4 NO 3 510°F Ta 4 X NH 4 NO 3 M 500 lb NH 4 NO 3 N 2O 2H 2O Ta 0 Example 1: Safety in Chemical Reactors Only liquid A in the vat as the product gases N2O and H2O escape immediately after being formed. dT dt Qg Qr N A C PA Q g ( r AV )( H Rx ) Q r F A 0 C PA (T T 0 ) B ( H B H B 0 ) UA (T T a ) 5 Unsteady State Energy Balance Q dT Q g r H Rx r A V FA 0 i C Pi T T 0 UA T T a N i C Pi dt Adiabatic Q r FA 0 C P A T 660 W 1134 CP W T 960 FA 0 0 dT dt H Rx rA V N iC P T i If the flow rate is shut off, the temperature 6 will rise (possibly to point of explosion!) t (min) Case 2 – Monsanto Chemical Company Keeping MBAs away from Chemical Reactors The process worked for 19 years before “they” showed up! Why did they come? What did they want? 7 Nitroanline Synthesis Reaction NO2 NO2 Cl NH2 + ONCB Chloride 8 + 2NH3 Ammonia + Nitroanaline NH4Cl + Ammonium Nitroanline Synthesis Reaction NH3 in H2O ONCB Autoclave 175 oC ~550 psi O-Nitroaniline Product Stream NH3 Separation Filter Press To Crystallizing Tanks 9 “fast” Orange Nitroanline Synthesis Reactor Old 10 3 kmol ONCB 43 kmol Ammonia 100 kmol Water V = 3.25 m3 Same Nitroanline Synthesis Reaction NO2 NO2 Cl NH2 + ONCB Chloride + 2NH3 Ammonia + Nitroanaline + NH4Cl Ammonium Batch Reactor, 24 hour reaction time Management said: TRIPLE PRODUCTION 11 MBA-Style: Nitroanline Synthesis Reactor New 12 9 kmol ONCB 33 kmol Ammonia 100 kmol Water V = 5 m3 Batch Reactor Energy Balance Qg dT dt NC P ( r A V )( H rx ) UA ( T T 0 ) N A 0 C pA N B 0 C pB N W C pW N A 0 C pA N B 0 C pB N W C pW dT dt 13 Qr Qg Qr NC p Batch Reactor Energy Balance dT Qg Qr dt NC p The rate of “heat removed” is UA Q r m c C Pc T a 1 T 1 exp m C c Pc Equation (12 - 13) p547 c , the maximum rate of heat removal is For high coolant flow rates, m Q r UA T Ta The rate of “heat generated” is Q g ( r A V ) H Rx r A V H Rx 14 rA k 1 C A C B Q g k 1 C A C B H Rx Batch Reactor Energy Balance Recall dT dT Qr Qg NC P S For isothermal operation at Qr = Qg, T = 448 K Q g k 448 K C A 0 1 X B X H Rx 2 15 Qr Qg cC P m c UA T a 1 T 1 exp m c C Pc 0 . 0001167 Vary m c to keep “heat removed” equal to “heat generation” C 2A 0 1 X Isothermal Operation for 45 minutes At the time the heat exchanger fails X 0.033, T 448 K Q g r AV H Rx 3850 kcal / min The maximum rate of removal at T 448 K is Q r UA T T a 35 . 85 ( 448 298 ) 5378 kcal / min Qr Qg Everything is OK Adiabatic Operation for 10 minutes t 45 min X 0 . 033 t 55 min X 0 . 0424 T 448 K T 468 K Q g 6591 kcal / min Q r 6093 kcal / min Qg Qr dT dt Qg Qr NC p 0 . 2 C / min Temperature-Time trajectory dT Temperature oC dt 0 . 2 C / min N Cp 400 Qr = 0 200 175 18 Qq Qr 9:55 t=0 Cooling Restored Isothermal Operation fuse 10:40 10:50 midnight 12:18 Disk Rupture The pressure relief disk should have ruptured when the temperature reached 265°C (ca. 700 psi) but it did not. If the disk had ruptured, the maximum mass flow rate out of the reactor would have been 830 kg/min (2-in orifice to 1 atm). vap H vap UA T T a Qr m Q r 449 , 000 Q g 27 , 460 kcal min kcal min Q r Q g No explosion All the following three things must have occurred for the explosion to happen 1. Tripled Production 2. Heat Exchange Failure 3.Relief Valve Failure 20 Case 3 – Manufacture of Fuel Additive methylcyclopentadiene manganese tricarbonyl (MCMT) 21 Production of methylcyclopentadienyl manganese tricarbonyl (MCMT). Step 1a. Reaction between methylcyclopentadiene (MCP) and sodium in a solvent of diethylene glycol dimethyl ether (diglyme, C6H14O3) to produce sodium methylcyclopentadiene and hydrogen gas: Step 1b. At the end of Step 1a, MnCl2 is added to the reactor. It reacts with sodium methylcyclopentadiene to produce manganese dimethylcyclopentadiene and sodium chloride: Step 1c. At the end of Step 1b, CO is added. The reaction between manganese dimethylcyclopentadiene and carbon monoxide produces the final product, methylcyclopentadienyl manganese tricarbonyl (MCMT), a fuel additive. 22 Only consider Step 1 Desired Reaction Undesired Reaction of Dygline Simplified Model Let A = methycylcopentadiene, B = sodium, S = Solvent (diglyme), and D = H2. These reactions are: (1) A + B C + 1/2 D (gas) r1A r1B k 1A C A C B (2) S 3 D (gas) + miscellaneous liquid and solid products r2 S k 2s C S H Rx1A 45 , 400 J mol 23 H Rx2S 3.2 10 5 J mol Case 3 – Manufacture of Fuel Additive 24 Case 3 – Manufacture of Fuel Additive 25 Case 3 – Manufacture of Fuel Additive (2) Rates Laws: Net Rates: 26 (3) Stoichiometry – Liquid Phase Case 3 – Manufacture of Fuel Additive (4) Energy Balance: 7 1.26 10 J K H Rx1A 45 , 400 J mol H Rx2S 3.2 10 27 5 J mol 28 End of Web Lecture 25 Class Lecture 2 29