Lecture 5
advertisement

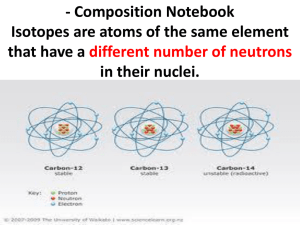
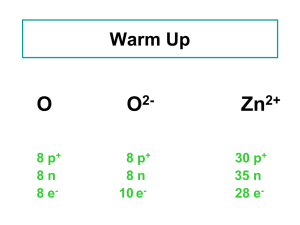
Lecture 5 Stable Isotopes Isotopes of Elements Types of Isotopes Chart of the Nuclides Measurements Delta Notation Isotope Fractionation Equilibrium Kinetic Raleigh See E & H Chpt. 5 Key questions: What are isotopes? What are the types of isotopes? How do we measure isotopes? How do we express measurements of isotopes? What is isotope fractionation and how do we express it? What is equilibrium isotope fractionation? What is kinetic isotope fractionation? What is Raleigh distillation? What are some applications of stable isotopes? Isotopes of Elements Atomic Number = # Protons = defines which element and its chemistry Atomic Weight = protons + neutrons = referred to as isotopes Different elements have different numbers of neutrons and thus atomic weights. Example: Carbon can exist as 12C, 13C, 14C How many protons and neutrons in each of the C isotopes? 12C = 6P, 6N 13C = 6P, 7N 14C = 6P, 8N 1 chemical, many isotopes! Where do Isotopes come from? In the beginning (Big Bang), light elements of H and He were formed (and a little bit of Li) Nuclear reactions (ie: fusion) in stars created the remaining elements (and are still creating), some of which have since decayed to more stable elements There are 92 naturally occurring elements – some are stable, some are not Types of Isotopes Isotopes can be categorized into 2 categories: Stable isotopes – Isotopes that do not decay over the timescale of earth history (4.5 billion years) Radioactive isotopes – Isotopes that spontaneously convert into other nuclei at a discernable rate The chart of the nuclides (protons versus neutrons) for elements 1 (Hydrogen) through 12 (Magnesium). Valley of Stability Most elements have more than one stable isotope. 1:1 line b decay X X a decay Number of neutrons tends to be greater than the number of protons Full Chart of the Nuclides 1:1 line Valley of Stability Examples for H, C, N and O: Atomic Protons Neutrons Weight (Atomic Number) Hydrogen H 1P 0N D 1P 1N Carbon 12C 6P 6N 13C 6P 7N 14C 6P 8N Nitrogen 14N 7P 7N 15N 7P 8N Oxygen 16O 8P 8N 17O 8P 9N 18O 8P 10N % Abundance (approximate) 99.99 0.01 98.89 1.11 10-10 1/2 = 5730 yr 99.6 0.4 99.76 0.024 0.20 % Abundance is for the average Earth’s crust, ocean and atmosphere Isotope Ratio Mass Spectrometer (IRMS) How we measure stable isotopes – the IRMS 1. Input as gases 2. Gases Ionized 3. Gases/ions accelerated in vacuum 4. Gases bent by magnetic field according to mass 5. Gases detected 1. 2. 5. 3. 4. Isotopes are measured as ratios of two isotopes. Standards are run frequently to correct for instrument stability Nomenclature – δ Notation Report stable isotope abundance as ratio to most abundance isotope (13C/12C) - Why? The ratio can be measured very precisely. BUT – any differences in the isotope ratio can be very very small so we use δ (“del”) notation dH sample ææ çç çç çè ç ç ç ç è H ö ÷ ÷ øsample ö ÷ ÷ østd = L H L æ ç ç è ö ÷ ÷ ÷ ÷ ÷ ÷ ÷ ø -1 ´1000 = æR ç sample çç R std è -1 ´1000 ö ÷ ÷÷ ø δ = “delta” or “del” (if you’re real savvy), units are per mil (‰) Where H = moles of heavy isotope L = moles of light isotope R = H/L δ tells us how much the sample deviates from the standard The sign of δ Compared to the standard Standards Vary Each standard has a well defined H/L ratio Example 1: The IRMS standard for C is PDB (13C/12C = 0.011237) Your sample has an 13C/12C = 0.010957. What is δ13C in ‰ for the standard? For the sample? Isotopic Fractionation • All isotopes of a given element have the same chemical properties • Small differences in the distribution of the isotopes in materials because heavier isotopes form stronger bonds and move slightly slower A heavier mass = stronger bond You would need a stronger string to hold two bowling balls together than you would need to hold two golf balls together Isotope Fractionation = process that results is differences in delta values in products and reactants Example: condensation of water vapor H2O(g) <=> H2O(l) In a closed water sample: δ 18O of H2O(g) = -1‰ (Atlantic) δ 18O of H2O(l) = -10‰ (Atlantic) Because of isotope fractionation! a and ε Nomenclature Fractionation Factor = a a= ææ çç çç çè çæ çç çç çè è H H L L ö ö ÷ ÷ ÷ ÷ ø product ÷ ÷ ö ÷ ÷ ÷ ÷ øreactant ÷ø a is unitless If a = 1, no fractionation If a >1, more heavy in product If a <1, more heavy in reactant Difference fractionation Factor = ε e =d H products dH - reactants If ε = 0, no fractionation If ε > 0 , more heavy in product If ε < 0 , more heavy in reactant ε is in permil (‰) e »1000´(a -1) Example #2: condensation of water vapor H2O(g) <=> H2O(l) In a closed water sample: δ 18O of H2O(g) = -10‰ δ 18O of H2O(l) = -1‰ What is ε and a of this reaction? Two kinds of Isotope Fractionation Processes 1. Equilibrium Isotope effects Occurs in equilibrium reactions (reactions can go both ways) if the system is in equilibrium Chemical equilibrium Phase changes (closed system) Distributes isotopes in a system so that the total energy of the system is minimized Heavier isotope equilibrates into the compound or phase in which it is most stably bound Within a molecule (CO2 vs HCO3-) Between molecules (CO2(g) vs CO2(aq) ) Usually applies to inorganic species. Usually not in organic compounds Due to slightly different free energies for atoms of different atomic weight Usually temperature dependent! Differences in vibrational energy is the source of the fractionation. Heavier isotopes wind up in the compound where it is bound more strongly Example #3: Condensation of Water Vapor in a closed container H2O(g) <=> H2O(l) H216O(l) + H218O(g) ↔ H218O(l) + H216O(g) In a closed container: δ18O of H2O(g) = -10‰ δ18O of H2O(l) = -1‰ a= ææ çç çç çè çæ çç çç çè è H H L L ö ö ÷ ÷ ÷ ÷ ø product ÷ ÷ ö ÷ ÷ ÷ ÷ øreactant ÷ø Is this reaction an example of an equilibrium isotope effect? How can you tell? Does the 18O “prefer” to be in the gas or liquid phase? Why? Example #4: Bicarbonate system The carbonate buffer system involving gaseous CO2(g), aqueous CO2(aq), aqueous bicarbonate HCO3- and carbonate CO32-. One step of that reaction: CO2(aq) + H2O ↔ HCO3- + H+ δ 13C of CO2(aq) = 1‰ a = 1.0092 at 0ºC and 1.0068 at 30ºC (The IRMS standard for C is PDB (13C/12C = 0.011237)) Is this reaction an example of an equilibrium isotope effect? How can you tell? What is the final δ13C of HCO3- at 0ºC at 30ºC? Is 13C more stable as CO2(aq) or HCO3-? Is there more or less fractionation at higher temperatures? 2. Kinetic Fractionation Occurs in unidirectional (irreversible) reactions reversible reactions that are not yet at equilibrium diffusion or differential bond breaking Heavier isotopes move more slowly (KE = ½ mv2) Therefore react more slowly Reaction products are depleted in the heavy isotope relative to the reactants All isotopes effects involving organic matter are kinetic Why do heavier isotopes move more slowly? Same kinetic energy, despite isotope E = ½ mv2 If E is the same and mass increases, the v must decrease Examples of Kinetic Fractionation Three types of kinetic fractionation: 1. Unidirectional reactions Example: Carbon fixation via photosynthesis: 12CO + H O -> 12CH O + O 2 2 2 2 faster 13CO + H O -> 13CH O + O 2 2 2 2 slower Organic matter gets depleted in 13C during photosynthesis (decreases in 13C) 2. Reversible reactions that are not yet at equilibrium Example: Evaporation of water vapor if not in equilibrium (net evaporation ie: N .Atlantic) H216O(l) -> H216O(g) faster H218O(l) -> H218O(g) slower Water vapor gets depleted in 18O during net evaporation (decreases in 18O) 3. Diffusion Example: Diffusion of H2Oacross a cell membrane H216O(l) outside cell -> H216O(l) inside cell faster H218O(l) outside cell -> H218O(l) inside cell slower Water vapor gets depleted in 18O during net evaporation (decreases in 18O) Equilibrium Fractionation vs Kinetic Fractionation The difference depends on the reason for the fractionation Equilibrium fractionation occurs so that the total energy of the system is minimized via forming the most stable bonds possible Equilibrium is related to bond stability of the isotope Kinetic fractionation occurs because smaller molecules move faster than heavier molecules and therefore react more slowly Kinetic is related to the speed of the isotope 13C in carbon reservoirs E & H Fig. 5.6 13C of atmospheric CO2 versus time See Quay, 1992, Science Raleigh Fractionation A combination of kinetic and equilibrium isotope effects • Kinetic when water molecules evaporate from sea surface (net evaporation b/c system is not in equilibrium) • Equilibrium effect when water molecules condense from vapor to liquid form A isotope fractionation reaction where products are isolated immediately from the reactants will show a characteristic trend in isotopic composition. Raleigh Fractionation - Concept • Vapor depleted in 18O compared to ocean water • Air masses transported to higher latitudes where it is cooler. • Rain enriched in 18O, removed from system (cloud) • Cloud gets lighter • Rain enriched in 18O, removed from system (cloud), but less enriched Raleigh Fractionation – Characteristic trend Example: Evaporation – Condensation Processes 18O in cloud vapor H2O(g) and condensate (H2O(l) rain) plotted versus the fraction of remaining vapor for a Raleigh process. Idealized: • 20ºC – All vapor -9‰ • Just colder than 20ºC – Condensate starts to form, more enriched in 18O, but is removed from the system (rained out) • The vapor continues to condense as the temperature decreases – becoming more and more depleted in 18O • Fractionation increases with decreasing temperature • Same pattern for D/H isotopes - different scale because more fractionation during the condensation (ε = +78‰ rather than +9‰) • This trend is used to reconstruct local paleotemperature from in Antartica and Greenland from ice cores 18O variation with time in Camp Century ice core. 18O was lower in Greenland snow during last ice age 15,000 years ago 18O = -40‰ 10,000 to present 18O = -29‰ Reflects 1. 18O of precipitation 2. History of airmass – cumulative depletion of 18O http://www.youtube.com/watch?v=nZC5EMPZDFA Applications of Stable Isotopes There are many applications of stable isotopes – especially in the study of past conditions on earth Three case studies in oceanography: 1. 2. 3. Paleothermometer from foraminifera shells Origin of organic matter Estimate primary production in marine systems Case study: 18O of forams in sediment to reconstruct paleotemperature HCO3- + Ca2+ ↔ CaCO3(s) + H+ Fractionation of 18O is temperature dependent and well quantified in labs The 18O of CaCO3 precipitated in forams reflects the temperature Preserved in marine sediments Complicated because although this relationship is well defined, depends on a known 18O of water . That may change due to ice volume. Case Study: Estimation of temperature in ancient ocean environments CaC16O3(s) + H218O CaC18O16O2 + H216O The exchange of 18O between CaCO3 and H2O The distribution is Temperature dependent last interglacial Holocene last glacial 18O of planktonic and benthic foraminifera from piston core V28-238 (160ºE 1ºN) Planktonic and Benthic differ due to differences in water temperature where they grow. Planktonic forams measure sea surface T Benthic forams measure benthic T Assumptions: 1. Organism ppted CaCO3 in isotopic equilibrium with dissolved CO322. The δ18O of the original water is known 3. The δ18O of the shell has remained unchanged Case study: 18O of forams in sediment to reconstruct paleotemperature Does the 18O of water in the ocean change over time? Large scale Raleigh distillation Net transfer of water from ocean to continental ice sheets make ice very depleted in 18O and the oceans enriched in 18O, increasing the 18O of water about 1‰ In some cores, pore water can be measured directly, which gets around this issue. Case study: 13C of bulk organic matter to determine source Many people are interested in the preservation in organic matter in marine sediments By looking at the 13C of an organic material, it can say something to how it was produced (marine or terrestrial) because the starting material is so different in 13C Complicated by C4 and CAM plants. C4 = grasses Crassulacean acid metabolism, also known as CAM photosynthesis, is a carbon fixation pathway that evolved in some plants as an adaptation to arid conditions Case study: Profiles of DI13C and 18O to estimate primary productivity The profiles of DI13C and 18O can be used to estimate primary productivity More photosynthesis in surface results in a heavier DI13C, resulting in a more positive 13C in surface DIC During respiration, 16O is preferentially taken up, resulting in a more positive 18O “left over” in the water (obvious at O2 minimum) Why does the 13C decrease slightly at the O2 minimum? North Atlantic data