Example 1
advertisement

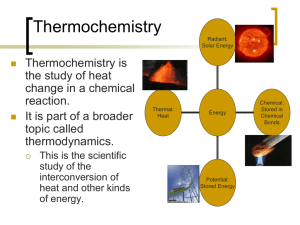
Thermochemistry Part 4: Phase Changes & Enthalpies of Formation Specific Heat Specific heat: The amount of heat that must be added to a stated mass of a substance to raise it’s temperature, with no change in state. q mc T Example: How much heat is released by 250.0 g of H2O as it cools from 85.0oC to 40.0oC? (Remember, specific heat of water = 4.18 J/goC) q = mcT q = (250.0 g)(4.18 J/goC)(40.0-85.0) q = -47,000 J = -47.0 kJ But what if there is a phase change? LATENT HEAT OF FUSION, Hfus Definition: the enthalpy change (energy absorbed) when a compound is converted from a solid to a liquid without a change in temperature. “Latent” means hidden; the heat absorbed/released during a phase change does not cause the temperature to change. Note: Hfus for water is 334 J/g LATENT HEAT OF FUSION, Hfus A = solid B = melting (solid + liquid) C = liquid D = boiling (liquid + gas) E = gas LATENT HEAT OF VAPORIZATION, Hvap Definition: the enthalpy change (energy absorbed) when one mole of the compound is converted from a liquid to a gas without a change in temperature. Note: for water Hvap is 2260 J/g LATENT HEAT OF VAPORIZATION, Hvap A = solid B = melting (solid + liquid) C = liquid D = boiling (liquid + gas) E = gas Example 1: How much heat is released by 250.0 g of H2O as it cools from 125.0C to -40.0C? Five steps… 1. Cool the steam 2. Condense 3. Cool the liquid water 4. Freeze 5. Cool the solid ice m∙csteam∙T m(-Hvap) m∙cwater∙T m(-Hfus) m∙cice∙T Example 1: How much heat is released by 250.0 g of H2O as it cools from 125.0C to -40.0C? When substances change state, they often have different specific heats: cice = 2.09 J/goC cwater = 4.18 J/goC csteam = 2.03 J/goC Example 1: How much heat is released by 250.0 g of H2O as it cools from 125.0C to -40.0C? cooling = exothermic → negative heat values qsteam = mcT = (250.0g)(2.03J/goC)(100.0–125.0) = -12,700 J qvap = mHvap = (250.0g)(-2260J/g) = -565,000 J qwater = mcT = (250.0g)(4.18J/goC)(0-100) = -105,000 J qfus = mHfus = (250.0g)(-334J/g) = -83,500 J qice = mcT = (250.0g)(2.09J/goC)(-40.0-0) = -20,900 J qtotal = -787,000J -787 kJ Now YOU try a few… Example 2: How much heat energy is required to bring 135.5 g of water at 55.0oC to it’s boiling point (100.oC) and then vaporize it? Water must be heated to it’s boiling point. q = mcT = (135.5 g)(4.18J/goC)(100-55) q = 25.5 kJ 2. Water must be vaporized: q = mHvap = (135.5 g)(2260 J/g) q = 306 kJ 3. Add them together: q = 25.5 kJ + 306 kJ = 332 kJ 1. Example 3: How much energy is required to convert 15.0 g of ice at -12.5oC to steam at 123.0oC? 1. 2. 3. 4. 5. Heat the ice Melt the ice Heat the water Vaporize the water Heat the steam. Example 3: How much energy is required to convert 15.0 g of ice at -12.5oC to steam at 123.0oC? qice = mcT = (15.0g)(2.09J/goC)(0.0 - -12.5) = 392 J qfus = mHfus = (15.0g)(334J/g) = 5,010 J qwater = mcT = (15.0g)(4.18J/goC)(100-0) = 6,270 J qvap = mHvap = (15.0g)(2260J/g) = 33,900 J qsteam = mcT = (15.0g)(2.03J/goC)(123.0-100.0) = 700. J qtotal = 392 J + 5,010 J + 6,270 J + 33,900 J + 700. J = 46,300J 46.3 kJ Enthalpies of Formation enthalpy H change (delta) f standard conditions formation Enthalpies of Formation usually exothermic see table for Hf value enthalpy of formation of an element in its stable state = 0 these can be used to calculate H for a reaction Standard Enthalpy Change Standard enthalpy change, H, for a given thermochemical equation is = to the sum of the standard enthalpies of formation of the product – the standard enthalpies of formation of the reactants. H rxn (H products f ) - (H sum of (sigma) reactants f ) Standard Enthalpy Change elements in their standard states can be omitted: 2 Al(s) + Fe2O3(s) 2 Fe(s) + Al2O3(s) ΔHrxn = (ΔHfproducts) - (ΔHfreactants) ΔHrxn = ΔHfAl2O3 - ΔHfFe2O3 ΔHrxn = (-1676.0 kJ) – (-822.1 kJ) ΔHrxn = -853.9 kJ Standard Enthalpy Change the coefficient of the products and reactants in the thermochemical equation must be taken into account: 2 Al(s) + 3 Cu2+(aq) ΔHrxn = 2 Al3+(aq) + 3 Cu(s) (ΔHfproducts) - (ΔHfreactants) ΔHrxn = 2ΔHfAl3+ - 3ΔHfCu2+ ΔHrxn = 2(-531.0 kJ) – 3(64.8 kJ) ΔHrxn = -1256.4 kJ Standard Enthalpy Change Example: Calculate H for the combustion of one mole of propane: C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(l) ΔHrxn = (ΔHfproducts) - (ΔHfreactants) ΔHrxn = [3ΔHfCO2+4ΔHfH2O] - ΔHfC3H8 ΔHrxn = [3(-393.5kJ)+4(-285.8kJ)]–(-103.8 kJ) ΔHrxn = -2219.9 kJ Standard Enthalpy Change Example: The thermochemical equation for the combustion of benzene, C6H6, is: C6H6(l) + 15/2 O2(g) 6CO2(g) + 3H2O(l) H = -3267.4 kJ Calculate the standard heat of formation of benzene. -3267.4kJ = [6(-393.5kJ)+3(-285.8kJ)]–ΔHfC6H6 -3267.4kJ = -3218.4–ΔHfC6H6 -49.0kJ = –ΔHfC6H6 ΔHfC6H6 = +49.0 kJ Standard Enthalpy Change Example: When hydrochloric acid is added to a solution of sodium carbonate, carbon dioxide gas is formed. The equation for the reaction is: 2H+(aq) + CO32-(aq) CO2(g) + H2O(l) Calculate H for this thermochemical equation. ΔH = [(-393.5kJ)+(-285.8kJ)]–[2(0 kJ)+(-677.1kJ) ΔH = (-679.3kJ)–(-677.1kJ) ΔH = -2.2 kJ