CP BIO: Ch. 7 The Cell Membrane - Northern Highlands Regional HS
advertisement

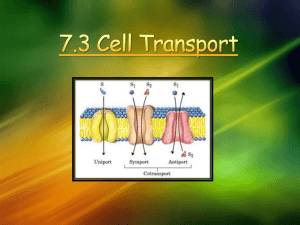
CP BIO: Ch. 7 The Cell Membrane and transport across the membrane Review: Protein structure Protein function needs 3-dimensional shape • Peptide bonds hold amino acids together polypeptide chain • Hydrogen bonds hold parts of chain together shape Protein Denaturation Denature – lose shape (and function) Chemical or physical changes - break bonds that hold the 3-D shape Denaturation can be caused by changes in ion concentration, pH, temperature, and others Energy in Chemical Reactions • Life processes are chemical • Need energy added (ACTIVATION ENERGY) to start reactions • Cells cannot use or make heat • ENZYMES speed up reactions – lower activation energy How enzymes work Enzymes are “biologic catalysts” catalyst - speeds reaction but is not changed or used up enzymes are specific – act on only one kind of molecule Names – for chemical process or substrate; - often end in -ase Enzyme must fit its target molecule • Enzymes function by binding to another molecule • Shapes must match, or bend to fit together Enzyme denatured – shapes do not fit Enzyme binds to Substrate • Substrate – molecule an enzyme acts upon • Active site – region on enzyme molecule that binds to substrate molecule - must fit together!! Active site substrate Enzymes catalyze all reactions A catabolic reaction Enzymes catalyze anabolic, too. ALL cell reactions need help of enzymes 2 models of enzyme fit 1. Lock and key - perfect match, no shape change 2. Induced Fit - shape of enzyme changes when substrate attaches Factors Affecting Enzyme Action 3-dimensional protein molecule - shape is critical Temperature - heat makes molecules move faster more contact between enzyme and substrate faster reaction rate BUT, high temps denature proteins! Enzymes and pH Work best at a specific pH - changes in pH break bonds holding molecule shape Enzyme Inhibition Sometimes other molecules stop enzyme action • Compete for/block the active site • Change active site by bonding somewhere else on the enzyme The Plasma membrane Boundary between the cell and its environment FLUID MOSAIC MODEL - Describes structure of cell membranes • “mosaic” – sea of lipids with scattered proteins • “fluid” – molecules float and move around within the layer Fluid Mosaic Model Phospholipid Phospholipid = lipid molecule with phosphate on one end • glycerol + two fatty acids + phosphate group • phosphate group = polar end (hydrophilic “head”) • Fatty acids = nonpolar end (hydrophobic “tails”) Phospholipids form a double layer Nonpolar tails are on the inside (away from water) Polar heads are on the outside ( touch water) Features of the Cell Membrane • Semipermeable = some substances can pass through - some cannot - depends on: molecule size, charge, polar or nonpolar, concentration, needs of the cell • Cholesterol – molecules scattered among the phospholipids - in animal cells - keep membrane flexible and stable • Carbohydrates – glucose chains on outside of cell - Identification “tags” Membrane Proteins have many functions Transport Enzyme Allows a specific molecule to pass through the membrane Catalyzes a reaction inside the cell Receptor Site for messenger molecule to attach Membrane proteins (2) Junctions Identification “SELF” – cell belongs Cells join to form tissues, in this organism, communicate immunity Structure Keeps internal parts organized How do membranes keep homeostasis? Cell membranes are selectively permeable • The lipid layer blocks most substances • Some molecules can cross the membrane – By passive or active transport • Some are too big to cross at all PASSIVE TRANSPORT USES NO CELL ENERGY Molecules move randomly – spread out until evenly distributed • From an area of higher concentration to an area of lower concentration Diffusion across membrane High Concentration Equal Concentrations Low Concentration Concentration gradient: two adjacent areas with different concentrations of a solute DIFFUSION Diffusion: movement of particles from an area of high concentration to an area of lower concentration • Particles move in all directions (random) • NET movement is from high concentration to low • “Down the concentration gradient” Diffusion • Particles spread out until evenly distributed equilibrium (homogeneous) • AT equilibrium, particles continue moving, but in all directions equally NO further change in concentration Cell Membranes allow some particles to cross Particles can diffuse across the lipid bilayer if they are: • Small • Nonpolar (lipid-soluble) Examples: CO2 , O2, fatty acids can diffuse easily FACILITATED DIFFUSION Transport proteins “help” particles move across the membrane – DOWN their gradient • PASSIVE transport – no cell energy used • Proteins are SPECIFIC – each allows only a certain substance to pass Transport Proteins • Pores and Channels – open path through membrane • Carrier proteins – take particle on one side of membrane and release on the other What crosses by Facilitated Diffusion? Particles can cross by facilitated diffusion if they are: • Small (monomers) • POLAR (water soluble) • or CHARGED (ions) – Examples: H2O, glucose, Ca+2, Cl- , Na+, K+ OSMOSIS Diffusion of water across a membrane Important process in cell homeostasis Water crosses the cell membrane easily - Small enough to pass between lipid molecules - Also pass through special proteins, aquaporins Why osmosis matters • Water crosses membrane easily, faster than many solutes • Will try to reach equilibrium • If NET water moves into or out of cell changes homeostasis • Unequal water on one side of cell membrane = osmotic pressure Osmotic pressure in cells ISOTONIC Equal concentrations of solutes inside and outside cell – Equal concentrations of water – Water goes in and out of cell at equal rates Isotonic pressure in cells • No NET movement of water into or out of cell • Normal water pressure in animal cells • Wilted – low water pressure in plant cells When solute concentration is different on two sides of a membrane lower solute concentration = hypotonic Higher solute concentration = hypertonic Solutes will move down their gradient IF THEY CAN CROSS THE MEMBRANE Water concentration is OPPOSITE of solutes Low solutes HIGH WATER concentration High solutes LOW WATER concentration Water WILL diffuse down its gradient and crosses the cell membrane easily Goes TO whichever side has more solutes Cells in hypertonic solutions Solutes are higher outside cell, water is lower Water leaves cell by osmosis Cytoplasm shrinks - “plasmolysis” - animal cells: shrivel - plant cells: low pressure - cytoplasm pulls away from cell wall - but cell wall does not shrink Cells in hypotonic solutions Solutes are lower outside cell, water is higher Water enters cell by osmosis Cytoplasm swells - animal cells: swell, may burst (“lyse”) - plant cells: high osmotic pressure “turgor” - won’t burst - cell wall - “Turgid” – stiff and firm, upright stem Osmosis in Animal Cells Animal cells like ISOTONIC conditions best Osmosis in Plant Cells Plant cells like HYPOTONIC conditions best Contractile Vacuoles Fresh-water protists (like Paramecia or Amoeba) must constantly remove water that comes into the cell by osmosis ACTIVE TRANSPORT – uses cell energy Two kinds: 1. Molecule transport against the gradient a. from low concentration to high b. pushed across by membrane proteins (pumps) c. uses energy ATP d. small molecules and ions Why would cells use active transport? 1) To concentrate substances: Examples: kidneys concentrate wastes in urine, - intestine concentrates nutrients in blood 2) To maintain an ion concentration - sodium-potassium pump – allows nerve impulses 2) Bulk Transport – uses energy To bring larger particles into or out of cell Endocytosis = brings material into cell - fold cell membrane around it form a vacuole a. Phagocytosis = “cell eating” - large particles or whole cells - examples: amoeba, white blood cells b. Pinocytosis = “cell drinking” - small folds of membrane take in liquids Pinocytosis – “cell drinking” Example: small intestine absorbs some water this way Exocytosis – send OUT of cell Vacuole containing substance fuses with cell membrane opens to outside of cell - for cell secretions - Examples: hormones from endocrine glands digestive juices from pancreas or intestine