Atomic Theory Class #5
advertisement

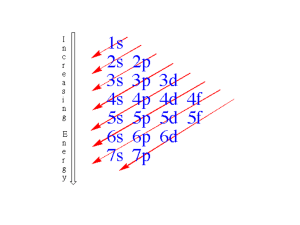
Atomic Theory Class #5 OB: full review of all important topics including… Isotopes, spectra, “the NY State Atomic Theory objective #1”, electron configurations, ground and excited states, maximum orbital size, the Gold Foil Experiment, Bohr’s Noble Prize, and the models of the atoms in order with their scientists. If you were to read the NY State Chemistry Curriculum, and you should, this is called objective 1 in Atomic Theory: The modern model of the atom has evolved over a long period of time through the work of many scientists. Pretty obvious, but worth repeating, long time, lots of mustaches. Lots. 2400 years, more mustaches than you can count. Line up the cast: 1 Democritus the Greek philosopher who gave us “atomos”, or in English, ATOMS 2 John Dalton the English Farmer, father of Atomic Theory (billiard ball model) 3 J. J. Thomson, the discoverer of the electron (plum pudding model) 4 Earnest J. Rutherford, discovered the nucleus, created first modern atomic model with electrons outside the nucleus, atom is mostly empty space 5 Niels Bohr, the physicist/mathematician/chemist who detailed the math to prove Rutherford’s model was correct. Proved electrons don’t lose energy, and live in orbits (oops), and that electrons can gain unique amounts of energy and jump to higher orbits, then back down (emitting spectra as that happens) 6 The Wave-Mechanical, or Modern model. Electrons no longer orbit the nucleus, rather they hang out in zones or ORBITALS, where you have a statistical chance of finding them most of the time (or not so much). Electrons act as bits of negative charged matter (mechanical), or as waves of energy, depending upon their “mood” (really) Dalton's Atomic Theory 1) All matter is made of atoms. Atoms are indivisible + indestructible. 2) All atoms of a given element are identical in mass and properties 3) Compounds are formed by a combination of 2 or more different kinds of atoms. 4) A chemical reaction is a rearrangement of atoms. There is no loss of atoms, nor new kinds of atoms produced in a chemical reaction. The one big problem with the Atomic Theory as outlined here? Think ISOTOPES You must be able to explain all of this: lead box, unstable isotope of polonium that emits alpha particle radiation, malleable gold foil, detection screen, positive nucleus, mostly empty space, dinging the nucleus, problems with the Rutherford model includes: no loss of energy for electron,+/- should attract, mostly empty space? The Bohr Model, you should be able to explain how orbits are also energy levels, ground state, excited state, how to become excited, what happens when electrons return to the ground state, maximum number of electrons per orbital (think group 18), Spectra, spectra lines, uses for spectra, problems with the “orbit” idea. Modern Models of atoms, shapes of the s, p, & d orbitals. Think ZONES, not orbits around the nucleus. Zones. These Spectra are “one half” of what you see when you wear the refractive lenses. They are standardized by size, and notice all of the numbers, this is NOT QUALITATIVE, it’s very quantitative. All spectra are measured and known, each atom/compound is different. More unique spectra, see the sodium, we saw that pair of yellow lines just before. Each spectra is mixed by our eyes so we see one color, lots of these colors appear “orange”, but with refractive lenses, we break up the mixture of light into the actual wavelengths of energy that make it up. Electron Configurations, ground state, excited state, when is spectra made? NO SPECTRA HERE (yet). When that electron drops back to the ground state orbital, it releases that unique amount of energy it took to get excited, as visible light. If you “break” the light mixture, you get bright line emission spectra, or spectra lines (different ways to say the same thing). Atom Ground state electron configuration Possible excited state configuration Number of electrons is always Mg – 12 2-8-2 2-7-3 12 Al – 13 2-8-3 2-7-4 13 C –6 2-4 1-5 6 Ca – 20 2-8-8-2 2-8-7-3 20 Atoms and their electrons gain energy lots of ways, heat, electricity, radiation, etc. They give off the gained energy as visible light. Spectra is ONLY produced when excited electrons give up their gained energy when they jump back to the lower energy ground state electron configuration. Excited atoms have the SAME NUMBER of electrons, just in different orbitals. KISS. Are these atoms in excited states correctly described, or not? If not, why not? Mg ground 2-8-2 Mg excited 1-9-2 F ground 2-7 F excited 2-8 He ground 2 He excited 1-1 Ne ground 2-8-8 Ne excited 2-8-7-1 Are these atoms in excited states correctly described, or not? If not, why not? Mg ground 2-8-2 Mg excited 1-9-2 X F ground 2-7 F excited 2-8 X He ground 2 He excited 1-1 YES Ne ground 2-8-8 Ne excited 2-8-7-1 X The Mg is wrong, only 8 electrons fit in n=2. F is wrong, it gained an electron when excited. Ne is wrong, that’s the configurations for argon! How many protons, neutrons and electrons are in Yttrium? How many protons, neutrons and electrons are in Yttrium? + (p Mass = 89 and n°) + At. # - 39 (just p ) 50 all that’s left is n° 39 protons means 39 electrons too. A new element Zanium, is discovered with 3 different isotopes. The mass and proportion of each is listed in the data table, what is the average weighted atomic mass for this new element? (don’t sweat the SF) Isotope Mass Naturally occurring proportion Za-49 Za-51 Za-54 49 amu 51 amu 54 amu 11.35% 4.65% 84.0% A new element Zanium, is discovered with 3 different isotopes. The mass and proportion of each is listed in the data table, what is the average weighted atomic mass for this new element? Isotope Mass Convert to decimal Mass x decimal = Za-49 (49 amu) (0.1135)= 5.5615 Za-51 (51 amu) (0.0465)= 2.3715 Za-54 (54 amu) (0.840)= 45.36 Average weighted atomic mass = 53.293 amu You should be able to define isotope, element, atom, compound, molecule, mixture, pure, heterogeneous, homogeneous, and all the vocabulary surrounding the models of the atoms. Read the Matter Diary, and the Atomic Theory Diary. Do the counting atoms drills, practice SF, memorize the models of the atoms in order, with their scientists and mustaches, be able to relate the gold foil experiment, calculate an average atomic mass from the isotope masses and proportions, and on and on. 30 multiple choice questions Friday, 3.3 points each, rounded to the nearest whole number. One period, but no real time limit. Bring reference tables, calculators, pens and pencils. Eat well, study but sleep too. Decide, a purple minivan, or waiting for the bus in the rain. Your choice.