Chemistry: Extended Experimental Investigation
advertisement

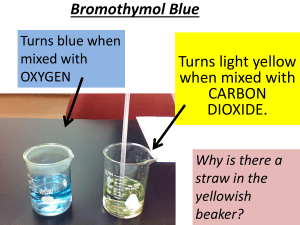
Practical Activity A – Preparation of Carbon Dioxide: Pre-lab Safety Information Material Hazard Control Magnesium carbonate Skin, eye and lung irritant Wear eye and skin protection; avoid breating dust Sodium hydrogen carbonate Slightly toxic if ingested Wear eye and skin protection 0.1 M hydrochloric acid Corrosive to eyes and skin Wear eye and skin protection Lime water (Ca(OH)₂) Slightly toxic if ingested; can burn skin and eyes Wear eye and skin protection Practical Activity B – Properties of Carbon Dioxide: Pre-lab Safety Information Material Hazard Control Methylated spirits Highly flammable Keep away from naked flame; wear eye and skin protection Universal indicator Irritant to eyes and skin Wear eye and skin protection Practical Activity C – Properties of Dry Ice: Pre-lab Safety Information Material Hazard Control Dry ice May cause severe burns Handle with adequately thick gloves and tongs Universal indicators Irritant to eyes and skin Wear eye and skin protection 0.1 M Sodium hydroxide Corrosive to skin and eyes Wear eye and skin protection Practical Activity D – Soda Water: acidity and effect on heating: Pre-lab Safety Information Material Hazard Control Universal indicator Irritant to eyes and skin Wear eye and skin protection Practical Activity E – Comparing cans of cola: Pre-lab Safety Information Material Hazard Control Lime water (Ca(OH)₂) Slightly toxic if ingested; can burn skin and eyes Wear eye and skin protection Boiling Coke May spontaneously explode; keep fair distance whilst boiling Wear eye and skin protection; wear gloves when handling 1. 2. 3. 4. 5. 6. Add about 3mL of Lime water (Ca(OH)₂) into a test-tube. Use a drinking straw to gently blow air through the solution until it becomes a cloudy state. Pour about 25mL of 10% glucose solution into a 100mL conical flask and then add as much yeast as can be accumulated onto a 10c piece. Stopper the flask with a single-holed stopper connected to a short length of glass tubing. Attach a 30cm rubber tubing and a glass delivery tube around 15 cm in length. Add around 10mL of lime water to a clean testtube and stand it in a test-tube rack with the flask of fermenting liquid. Insert the delivery tube from the flask into the lime water test-tube. Set the apparatus aside in a room for around 1-2 days. Note the odour of the fermenting liquid. Record your observations. CHEMICAL REACTIONS Experiment: A Experiment: B CO2(G) + CaOH2(L) CaCO3(S) + H2O (L) CaCO₃ (s) CaO (s) + CO₂ CO₂ (g) + H₂O (l) CO₂ (g) +H₂O (l) (g) H₂CO₃ (aq) H₂CO₃ (aq) How dissolved CO₂ makes soda water acidic CO₂ reacting with Ca(OH)₂. C₆H₁₂O₆ (aq) C₂H₅OH (aq) + 2CO₂ (g) Production of CO₂ from fermented glucose. MgCO₃ (s) MgO (aq) + CO₂ (g) Prod. Of CO₂ from decomposition of magnesium carbonate by heat. NaHCO₃ (s) + HCl ( ) NaCl (aq) + CO₂ (g) +H₂O (l) Prod. Of CO₂ from addition of hydrochloric acid to sodium hydrogen carbonate. CH₄ (g) + 2CO₂ (g) C (g) + 2H₂O (g) When air hole of bunsen burner is closed. CH₄ (g) + 2CO₂ (g) CO₂ (g) + 2H₂O (g) When bunsen burner is open. CaCO₃ (s) + 2HCl (aq) CaCl₂ (aq) + H₂O (l) + CO₂ (g) Prod. Of CO₂ in Kipp’s apparatus. CO₂ (g) + Ca(OH)₂ (aq) CaCO₃ (s) + H₂O (l) After the solution is left to settle, calcium carbonate forms back in the reaction. Experiment: C Experiment: D Experiment: E CaO (aq) + CO₂ (g) CaCO₃ (s) One of the greatest use of carbon dioxide is as a chemical in the production of carbonated beverages; produces the sparkle and fizz in beverages such as soda water. Carbon dioxide is also formed by the activity of yeast or baking powder, which is the logical reason why bread dough rises whilst heated in the oven. In fire extinguishers, large amounts of carbon dioxide is compacted in the cylinder and ejected through the nozzle and settles on flames; suffocating the flame and putting it out. The solid form of carbon dioxide, known as ‘dry ice’, is used as a refrigerating cooling-agent or for producing mist. The most common use of carbon dioxide by all plant life is in the process known as ‘photosynthesis’, in which they produce their food with glucose and the byproduct being oxygen. Carbon dioxide plays an important role in Earth’s greenhouse effect as it regulates Earth’s temperature by keeping the sun’s heat inside Earth’s atmosphere. However, due to human industrial activities and such, carbon dioxide has accumulated and increased over the many years and is genuinely ‘absorbing’ too much of the sun’s heat. Human activities that have contributed to Earth’s atmospheric issues: - Burning of fossil fuels: Major issue with carbon dioxide outbreak as fossil fuels have been used to produce electricity and for everyday use by humans for purposes such as; transportation, technological use, etc. - Deforestation: Clearing of forests and trees have contributed to decreasing photosynthetic plants and disrupting the balance of oxygen/carbon dioxide in the atmosphere. Less trees = more carbon dioxide. Chemistry Practical Booklet – Unit 2 Practical Activity A-E – Carbon Dioxide http://www.infoplease.com/ce6/sci/A0810371.html Information on some uses of carbon dioxide http://www.uigi.com/carbondioxide.html Facts on carbon dioxide – includes statistics http://www.blurtit.com/q137497.html Simplified, basic uses of carbon dioxide http://www.lenntech.com/carbon-dioxide.htm Environmental issues with carbon dioxide