Document
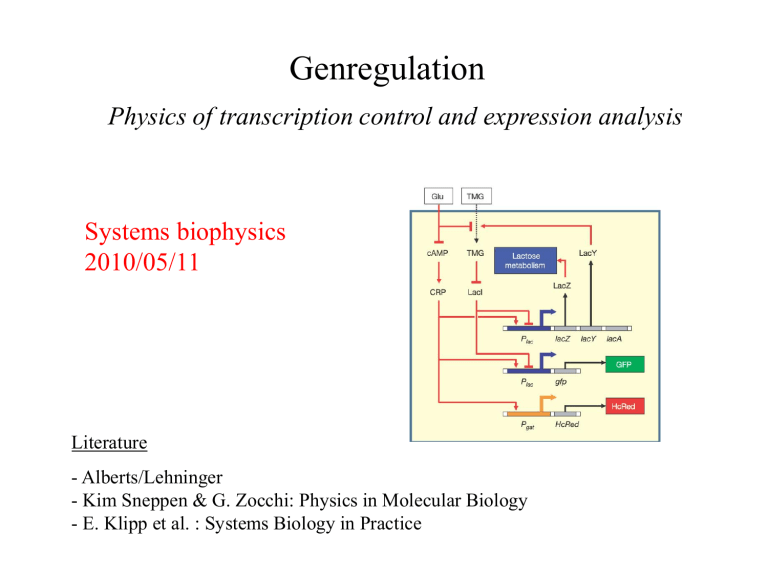
Genregulation
Physics of transcription control and expression analysis
Systems biophysics
2010/05/11
Literature
- Alberts/Lehninger
- Kim Sneppen & G. Zocchi: Physics in Molecular Biology
- E. Klipp et al. : Systems Biology in Practice
From genetic approach to sytemic approach
DNA mutations / evolution genregulation mRNA regulation protein functions signal transduction spatiotemporal structure formation
Morphogenesis
=> Topics of systems biophysics
Biological Pattern formation and Morphogenesis
Reaction-Diffusion-Model of Morphogenesis
11.05.2010
Zur Anzeige wird der QuickTime™
Dekompressor „TIFF (LZW)“ benötigt.
Biochemical Network
Enzymatic Reactions
Michaelis-Menton-Kinetics
Inhibation, Regulation
E
E.coli as model system
Genregulation allows adaption to changing environmental conditions, and regulation of metabolism
E.coli has a single DNA molecule which is 4.6 10 6 basepairs long. It encodes 4226 proteins and a couple of RNA molecules. The information content of the genome is is bigger than the structural information of the encoded Proteins
-> regulatory mechanisms are encoded
Content of this lecture:
Basics: Monod Model, Lac Operon
Statistical Physics of DNA-binding Proteins
Modelling of genregulatory Networks
(ODE & Boolian Networks)
Dynamics of Protein-DNA binding
DNA looping
Analysis of gene expression data
Synthetic Networks
Operon-Modell
Francois Jacob und Jaques Monod, 1961 operon
Operon: Genetic subunit, that consists of regulated genes with similar functionality.
It includes
- Promotor: Binding site for RNA polymerase
- Operator: controls access of the RNA-Polymerase structural gene
- Structural genes: Polypeptide encoding genes
The Trp Operator as a switch:
• Within the promotor lies a short DNA region as binding site for a repressor.
A bound repressor prevents the Polymerase from binding.
Small channel
The OUTSIDE of proteins can be recognized by proteins
Distinct basepairs can be recognized by their margins DNA binding motivs
Large channel
Binding of Tryptophane to the Tryptophane-Repressorproteine changes the conformation of the repressor,
Repressor can bind to the repressor binding site
Identification of promotor sequences
Transcription-Activation proteins switch on genes
Gen-Regulation with Feedback: lac -Operon
IPTG, TMG
LacI
Non-metabolizable inducer are used to induce gene expression
IPTG ( Isopropyl β-D-1-thiogalactopyranoside )This compound is used as a molecular mimic of allolactose , a lactose metabolite that triggers transcription of the lac operon . Unlike allolactose, the sulfur (S) atom creates a chemical bond which is non-hydrolyzable by the cell, preventing the cell from "eating up" or degrading the inductant. IPTG induces activity of betagalactosidase , an enzyme that promotes lactose utilization, by binding and inhibiting the lac repressor. In cloning experiments, the lacZ gene is replaced with the gene of interest and IPTG is then used to induce gene expression.
A cis-regulatory element or cis-element is a region of DNA or RNA that regulates the expression of genes located on that same strand. This term is constructed from the Latin word cis , which means "on the same side as". These cis-regulatory elements are often binding sites of one or more trans-acting factors.
Campbell, N.A., Biology
Variation of Protein-Concentration with IPTG
Northern Blot: measurement of the messenger RNA (mRNA) concentration
60
Long, C et al, J.Bacteriol. 2001
40
20
0
0.00
[IPTG Induktor]
0.10
External and internal Inductor-concentration is equal in equilibrium
The mRNA concentration increases linear with the concentration of inductor, saturation over 60%
The operon enables a variation of Protein concentration. What is missing to make a switch?
Transkription und Translation in E.coli
Typical times and rates
1 Molecule / cell = 1nM
Complete mass2.5 10 6 Da
TRANSKRIPTION rate 1/s - 1/18s
Transkriptionsrate: 30bps-90bps
TRANSLATION
10.000-15.000 Ribosomes
Translation rate 6-22 codons/s
(40 Proteine/mRNA)
pBAD24 2
~55 copies/cell
The arabinose system 1
Reporter
Break down
Regulator
[ 1] R. Schleif. Trends in Genetics, 16(12):559 –565, 2000
[2] L. M. Guzman, D. Belin, M. J. Carson, and J. Beckwith. J.Bacteriol., 177(14):4121 –4130, 1995
[3] D. A. Siegele and J. C. Hu. Proc. Natl. Acad. Sci. USA, 94(15):8168 –8172, 1997
Uptake
t n
Time-lapse Fluorescence Microscopy and Quantitative Image
Processing t
1
Fluorescence t
0
DIC t n
DIC t
0 automated data aquisition define ROIs measure total intensity
N background correction calibration and conversion into molecular units
Judith.Megerle@physik.lmu.de
8x10
5
Single cell expression kinetics
Saturating induction
0.2% arabinose
Fluorescence measurement
• Cell outlines are determined using bright field images
• The signal is integrated within the outline in each fluorescence image
6
4
2
0
0 20 60 80
5min 15min 25min 35min 45min
40
Time [min]
Subsaturating induction
30min 40min 50min 60min 70min
8x10
5
0.01% arabinose
6
4
2
0
0 20 40
Time [min]
60 80
Image series correspond to blue curves
Gene expression model
Reporter module
Deterministic rate model
Uptake module with time delay d
Induction: t=0min
8x10
5
6
4
2
0
0 20 40
[min]
60 80
Curve Fitting
Fit expression function
Fixed Parameters
Literature
Measured
Saturating induction
8x10
5
0.2% arabinose
6
4
2
0
0 20 40
Time [min]
Subsaturating induction
60
Fit Parameters
Time delay
Protein synthesis rate
8x10
5
0.01% arabinose
6
4
2
0
0 20 40
Time [min]
60
80
80
Ohter example: Quorum Sensing
Squid with floodlamp
Phänomena:
Squid ( Euprymna scolopes ) emmits light due the night
Squid isn ´ t recognized as prey in the moonlight
Explanation:
Light organ of the squid collects luminescent bacteria ( Vibrio fischerei )
Question:
Why does V. fischerei emmit light within the lightorgan of the squid, but not in open sea?
Quorum sensing
Bacteria increase exponential
OD: optical density
K. Nelson,
Cell-Cell Signalling in Bacteria
Bakterien detect their own cell density
Density regulates the expression of luminescent genes
Molekular picture of QS
• Bakteria export oligopeptides (Pheromones)
• Oligopeptides accumulate with increasing cell density
• Oligopeptide diffuse into cell membrane and regulates the expression of luminescent genes
Searching the binding site
Searching the binding site: timescales
D kT
6 R
Stokes Einstein equation
(z.B. D
GFP
=37µm 2 /s)
P , t )
1
exp tD
r
4
Probability distribution
1µm d 2 t
2 D
Typical timescale for a proteine to find an arbitrary point in an E.coli: t
D
0.1s
Diffusion to a target site (binding disc)
J D
r 2 dC dr dt
1 r 2
D d dr
4
2
C r )
J
r
C
) V
0
J 4 D
N
V
on
4
V
N
20
Residence times for transcription factors
on
for specific bindings (operon) with 1M -1
G spez
=-12.6kcal/mol,
=1 follows
=1.6nm
3
off
s and
(from
on
=20s/N follows, that 1 molecule in 1µm 3 occupies half an Operator)
for unspecific binding sites with
G uspez
=-10 -4 kcal/mol, follows
off
s
Search of the binding sites on a DNA strand
Unspecific binding events of TFs is a problem, since the time to find a binding site is increased. For a infinite staytime, a 1D- random walk over the strand would last:
L
2
2 D
1
200 .
000 s
2 Days (L=1.5mm und D
1
≈D)
Accelerated search: jumps between strands decrease time to find a binding site.
l 2
D
L
l
Ll
Mit L=1.5mm, l=150nm follows
Boolian Networks, or what cells and computers have in common.
( Nature, Dec 99)
Combinatoric gene regulation: Genetic networks
Genregulatoric proteine translation transcription
A transcription-activator and a transcription-repressor regulate the lac-Operon
Thermodynamicc model of a combinatoric transcription logics
Gene regulation follows the mechanics of
„Boltzmann-machines“
P : binding probability
Gerland et al. PNAS, 2005
Statistical physics of protein - DNA binding
K
k
k
O
Binding-isothermes:
K
Cooperativity due to dimer binding
K
D
M
D
2
Cooperative binding
K
K
The statistical weight of the „on“ state
P on
Z ( on
Z
P
c
K
The free-energy difference is normalized to 1mol/l . The real change in free energy of the binding event depends on the concentration of TF in
kT
A model for lac networks
Glukose conc.
constant
GFP: Reportermolekül, Abbildung durch
Fluoreszenz-Mikroskopie
=> je höher das Fluoreszenz-Signal desto mehr LacZ,Y wird exprimiert
Experimental proof for a switch
Start: not induced
After induction exist 2 populations: green: induced bacteria white, not induced population
Bistable area (grey)
Arrow marks the start state: on-off state of bacteria depend on the on-off state in the beginning!
switch with hysteresis
Ozbudak et al, Nature 2004
modelling of genregulatory networks: example
Modelling in mRNA level
Timetrace of mRNA concentrations
Steady state
Problem: kinetic binding constants are usually not known and hard to measure
Simplification of genregulatory networks
Genregulatory protein translation transcription
Abstraction of genetic networks
Gen X
+
-
Gen Y
Gen Z
Boolean networks
(Kauffman 1989)
Boolean networkmodel
• N Genes (nodes)
• with 2 N different states
2 2 K
• with possible rules
• K is the number of possible inputs per node
Boolean rules for N=2 und K=2
Back to the example:
We learn: if a=0, then follows
0101 stationary if a=1, then follows oscilatory behaviour
1000->1001->1111->1010
->1000