Src
advertisement

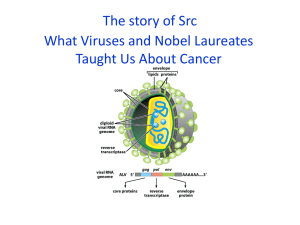
The story of Src What Viruses and Nobel Laureates Taught Us About Cancer Before We Start--remember you’ll be reading and be ready to discuss a paper next week How to read a Paper for the paper, as well as for each figure 1) What did we already know? 2) What is the question addressed here? 3) How did the researchers address this question? 4) What are the results? 5) What are the conclusions? No thank you! Peyton Rous discovered a virus that causes cancer in chickens The Rous Sarcoma Virus (RSV) A virus can transform a normal cell into a tumor The Rous Sarcoma Virus (RSV) A virus can transform a normal cell into a tumor Nobel Prize in Physiology or Medicine 1966 The Rous Sarcoma Virus (RSV) A virus can transform a normal cell into a tumor But what’s a virus??? Carcinogens Chemicals can directly induce cancer 1920s Viral Infection Out Yamagiwa Chemical Induction In 30 Years Later: Rebirth of RSV research RSV can transform cells in culture RSV stock Howard Temin Harry Rubin Immortality Studying cancer at the cellular level RSV infection Changed cells No contact inhibition on cell division No contact inhibition of cell division Normal Normal RSV infected = Cancer RSV infected = Cancer But how??? Normal Normal RSV infected = Cancer RSV infected = Cancer I HOPE you remember the central dogma This is one of those biology facts That you need to have permanently stored The central dogma DNA Transcription mRNA Translation Protein RSV is a retrovirus These viruses reverse the central dogma, making a DNA copy of their RNA genome and inserting it into your DNA Alberts et al. Fig. 24-23 Nobel Prize in Physiology and Medicine 1975 Howard Temin and David Baltimore Alberts et al. Fig. 24-23 Your genome is a retrovirus graveyard: living and dead retroviruses make up 8%of your genome, with ~100,000 whole or partial copies! Alberts et al. Fig. 24-23 NEXT Breakthrough discovery Retroviruses can cause cancer by picking up mutated versions of normal cellular genes Alberts et al. Fig. 24-23 The paper that created two more Nobel laureates and founded the modern field of Cancer biology Let’s take a very short detour Retroviruses can also cause cancer by inserting next to and thus activating the expression of proto-oncogenes Retroviral insertion sites in different tumors Transcribe to mRNA 5 kilobases Alberts et al. Fig. 22-24 exons wnt-1 gene Two mechanisms of gene activation by retroviral insertion Lodish et al. Fig. 24-10 OK—Back to src You know mis-expressing this gene can Initiate cancer What do you want to know now?? So, what job does the protein encoded by src do within the cell? The first BIG step: using antibodies to immunoprecipitate the v-Src protein This led to the discovery that Src is post-translationally modified This led to the discovery that Src is post-translationally modified What’s translation?? Protein kinases and protein phosphatases add and remove phosphate groups from target proteins Lodish et al. Fig. 20-5 Adding labeled ATP to a precipitated Src showed that Src can phophorylate a substrate Src is a kinase in the presence of P32-ATP A substrate is phosphorylated Which amino acids can be phosphorylated? And Why those amino acids?? Src is a Tyrosine Kinase As a kinase, it can affect many cellular events Figure 15-18a Molecular Biology of the Cell (© Garland Science 2008) Normally, Src kinase intrinsic activity is low What makes Src so active in transformed cells? Western Blot with antibody that recognizes Tyr phosphorylated proteins The structures of c-src and v-src provided an important clue! Lodish et al. Fig. 24-17 Src contains three domains that are shared with other proteins Binds polyproline motifs Phosphorylates other proteins Binds peptides phosphorylated on Tyr Scientists have determined the precise 3-dimensional structure of Src Xu et al. Nature. 1997 385:595-602 Tyrosine phosphorylation of the C-terminus creates an intramolecular and inhibitory interaction Lodish et al. Fig. 24-17 Src is normally inactive due to intramolecular inhibition Lodish et al. Fig. 24-17 Recent work has provided a more detailed model of Src activation Closed = OFF Open = ON Cowen-Jacob et al. Structure 13, 861-871 (2005) v-src lacks the C-terminal Tyr and thus cannot be inactivated! Lodish et al. Fig. 24-17 Activation of Src has multiple consequences From Schwartzenberg, Oncogene 17, 1463-1468 (1998) Where is Src within cells? This is a covalently attached lipid what might that mean? Myristylation of Src is essential for transformation Recent work has provided a more detailed model of Src activation Cowen-Jacob et al. Structure 13, 861-871 (2005) c- Src is a tyrosine kinase What does it do in the cell? What are its targets? Remember, we are still in the late 70s Bishop and Varmus Identifying The Targets of Src-look for Proteins ONLY modified by biologically active Src Western blotting with antiphosphotyrosine antibodies V = v-Src transfected cells 2A/V = non-myristylated v-Src transfected cells Reynolds et al. MCB (1989) Identifying The Targets of Src-look for Proteins ONLY modified by biologically active Src Western blotting with antiphosphotyrosine antibodies V = v-Src transfected cells 2A/V = non-myristylated v-Src transfected cells p120 catenin: modulates cellcell adhesion Reynolds et al. MCB (1989) Identifying the targets of Src - p120 catenin: modulates cell-cell adhesion - Cortactin A: regulates actin polymerization - Focal Adhesion Kinase: involved in cell-matrix interactions Mike Schaller, ex-UNC Src modulates both cell-cell and cell matrix adhesion: The basics Cell-cell junctions Cell-matrix junctions Basal lamina Src modulates both cell-cell and cell matrix adhesion: The basics Lodish et al. Fig. 22-2 Epithelial cells secrete a special ECM called the basal lamina Epithelial cells Basal Lamina Alberts et al. Fig. 19-54 Cells interact with the ECM via Focal adhesions, which also anchor the actin cytoskeleton Focal Adhesions (orange) Actin: Green Alberts et al. Fig. 17-42 Focal adhesions are linked to the actin cytoskeleton Alberts et al. Fig. 16-75 A complex network of proteins links the focal adhesion to actin and regulates actin polymerization Alberts et al. Fig. 16-75 Focal adhesions are sites of intense protein tyrosine phosphorylation Focal adhesions Actin: Green Phosphotyrosine: Red An oversimplified model of Src function Normal skin cell tightly adherent to ECM Wounding->platelet recruitment-> cell migration and proliferation Alberts et al. A less oversimplified model Migratory growth factors e.g., EGF, PDGF RTKs Src FAK PI-3kinase Extracellular matrix Integrins Adaptors Actin Remodel cell-matrix junctions -> cell motility From Jones et al. Eur J. Cancer 36, 1595-1606 (2000) FAK is recruited by integrins to the membrane and is autophosphorylated - Src binds to phosphorylated FAK - Src changes conformation and becomes active - Src further phosphorylates FAK - Src-FAK phosphorylate target proteins Src and FAK act together to regulate other focal adhesion proteins Src-FAK signals to regulate adhesion turnover Src-FAK active = less adhesion, more migration If Src is a critical regulator of cell adhesion, what happens to an animal without any Src? Cell 1991 64:693-702 Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Soriano P, Montgomery C, Geske R, Bradley A. Why is this phenotype so modest? Redundancy!! Src has two very close relatives: Fyn and Yes Different Src family kinases work downstream of different receptors Alberts et al. Fig. 23-54 Fyn mutant mice are viable but have defects in myelination of brain neurons Yes mutant mice are viable but with subtle changes in B-cell function Src; Fyn; Yes triple mutant mice die at embryonic day 9.5 with multiple defects Triple mutant Wild-type However, triple mutant cells still make focal adhesions However src; fyn; yes (SYF) triple mutant cells fail to migrate! Scratch assay Scientists have determined the precise 3-dimensional structure of Src Active site This aided identification of kinase inhibitors that block Src action SU6656 Active site In leukemia, adding Src inhibition to inhibition of the related kinase Abl improves prognosis in phase II trials. It is approved to help get around drug resistance in CML dasatinib Ottmann et al. Blood 110, 2309 (2007) This same Src inhibitor is in Phase II trials for advanced breast cancer, melanoma and advanced sarcomas dasatinib Ottmann et al. Blood 110, 2309 (2007) Another Src inhibitor is in Phase I/II trials for metastatic pancreatic, breast, ovarian, and prostate cancers Active site AZD0530