CHAPTER 6
advertisement

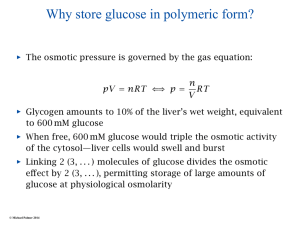
Chapter 22 Gluconeogenesis, Glycogen Metabolism, and the Pentose Phosphate Pathway Biochemistry by Reginald Garrett and Charles Grisham Outline of Chapter 22 1. What Is Gluconeogenesis, and How Does It Operate? 2. How Is Gluconeogenesis Regulated? 3. How Are Glycogen and Starch Catabolized in Animals? 4. How Is Glycogen Synthesized? 5. How Is Glycogen Metabolism Controlled? 6. Can Glucose Provide Electrons for Biosynthesis? 22.1 – What Is Gluconeogenesis, and How Does It Operate? • • • • Generation (genesis) of "new (neo) glucose" from common metabolites Humans consume about 160 g of glucose per day, 75% of that is in the brain Body fluids contain only 20 g of free glucose Glycogen stores can provide 180-200 g of glucose So the body must be able to make new glucose from noncarbohydrate precursors— gluconeogenis Substrates for Gluconeogenesis Pyruvate, lactate, glycerol, amino acids and all TCA intermediates can be utilized • Fatty acids cannot! Why? – Most fatty acids yield only acetyl-CoA – Except fatty acids with odd numbers of carbons • Acetyl-CoA (through TCA cycle) cannot provide for net synthesis of sugars in animal, but plants do! Why? (chap 19) Substrates for Gluconeogenesis 1. 2. 3. 4. Lactate Amino acids Glycerol Propionyl-CoA FAs with Odd numbers of C Gluconeogenesis • • Occurs mainly in liver (90%) and kidneys (10%) Not the mere reversal of glycolysis for 2 reasons: 1. Energetics: must change to make gluconeogenesis favorable (DG of glycolysis = -74 kJ/mol) 2. Reciprocal regulation: Gluconeogenesis is turned on, and when glycolysis is turned off, and vice versa The new reactions provide for a spontaneous pathway (DG negative in the direction of sugar synthesis), and they provide new mechanisms of regulation Something Borrowed, Something New 1. Seven steps of glycolysis are retained: Steps 2 and 4-9 2. Three steps are replaced: Steps 1, 3, and 10 (the regulated steps!) Figure 22.1 The pathways of gluconeogenesis and glycolysis. Species in blue, green, and peach-colored shaded boxes indicate other entry points for gluconeogenesis (in addition to pyruvate). Hexokinase Phosphofructokinase 1. Pyruvate Carboxylase Pyruvate is converted to oxaloacetate • The reaction requires ATP and bicarbonate as substrates • Biotin is covalently linked to a lysine residue at the active site and as a coenzyme • The mechanism is typical of biotin carbonylphosphate pyruvate carbanion Figure 22.3 A mechanism for the pyruvate carboxylase reaction. Bicarbonate must be activated for attack by the pyruvate carbanion. This activation is driven by ATP and involves formation of a carbonylphosphate intermediate—a mixed anhydride of carbonic and phosphoric acids. (Carbonylphosphate and carboxyphosphate are synonyms.) • The conversion is in mitochondrial matrix • Acyl-CoA is an allosteric activator • Regulation: – If levels of ATP and/or acetyl-CoA are low Pyruvate is converted to acetyl-CoA Enters TCA cycle – If levels of ATP and/or acetyl-CoA are high Pyruvate is converted to oxaloacetate Enters gluconeogenesis glucose • Pyruvate carboxylase is found only in mitochondrial matrix • Oxaloacetate cannot be transported across the mitochondrial membrane Figure 22.4 Pyruvate carboxylase is a compartmentalized reaction. Pyruvate is converted to oxaloacetate in the mitochondria. Because oxaloacetate cannot be transported across the mitochondrial membrane, it must be reduced to malate, transported to the cytosol, and then oxidized back to oxaloacetate before gluconeogenesis can continue. 2. Phosphoenolpyruvate Carboxykinase Conversion of oxaloacetate to PEP • Lots of energy is needed to drive this reaction • Energy is provided in 2 ways: 1. Decarboxylation is a favorable reaction and helps drive the formation of the very high-energy enol phosphate 2. GTP is hydrolyzed, GTP used here is equivalent to an ATP PEP Carboxykinase • The overall DG for the pyruvate carboxylase and PEP carboxykinase reactions under physiological conditions in the liver is -22.6 kJ/mol • Once PEP is formed in this way, the other reactions act to eventually form fructose-1,6bisphosphate 3. Fructose-1,6-bisphosphatase Hydrolysis of F-1,6-bisP to F-6-P • Thermodynamically favorable - DG in liver is -8.6 kJ/mol • Allosteric regulation: – citrate stimulates – fructose-2,6-bisphosphate inhibits – AMP inhibits (enhanced by F-2,6-bisP) 4. Glucose-6-Phosphatase Conversion of Glucose-6-P to Glucose • G-6-Pase is present in ER membrane of liver and kidney cells • Muscle and brain do not do gluconeogenesis • G-6-P is hydrolyzed after uptake into the ER Figure 22.5 Glucose-6-phosphatase is localized in the endoplasmic reticulum. Conversion of glucose-6-phosphate to glucose occurs following transport into the ER. • The glucose-6-phosphatase system includes the phosphatase itself and three transport proteins, T1, T2, and T3. • T1 takes glucose-6-P into the ER, where it is hydrolyzed by the phosphatase • T2 and T3 export glucose and Pi, respectively, to the cytosol • Glucose is exported to the circulation by GLUT2 Gluconeogenesis Inhibitors and Other Diabetes Therapy Strategies Diabetes is the inability to assimilate and metabolize blood glucose • Metformin improves sensitivity to insulin by stimulating glucose uptake by glucose transporters • Gluconeogenesis inhibitors may be the next wave of diabetes therapy • 3-Mercatopicolinate and hydrazine inhibit PEP carboxykinase • Clorogenic acid (natural product found in the skin of peaches) inhibits transport activity by the glucose-6phosphatase system. S-3483 does the same, but binds a thousand times more tightly to the transporter Lactate Recycling – Cori cycle Lactate formed in muscles is recycled to glucose in the liver • Recall that vigorous exercise can lead to a buildup of pyruvate and NADH, due to oxygen shortage and the need for more glycolysis • NADH can be reoxidized during the reduction of pyruvate to lactate • Lactate is then returned to the liver, where it can be reoxidized to pyruvate by liver LDH • Liver provides glucose to muscle for exercise and then reprocesses lactate into new glucose (about 700) Figure 22.7 The Cori cycle. 22.2 – How Is Gluconeogenesis Regulated? Reciprocal control with glycolysis • When glycolysis is turned on, gluconeogenesis should be turned off, and vice versa – When energy status of cell is high, glycolysis should be off and pyruvate and other metabolites are ustilized for synthesis (and storage) of glucose – When energy status is low, glucose should be rapidly degraded to provide energy • The regulated steps of glycolysis are the very steps that are regulated in the reverse direction Figure 22.8 The principal regulatory mechanisms in glycolysis and gluconeogenesis. Activators are indicated by plus signs and inhibitors by minus signs. Allosteric and Substrate-Level Control • Glucose-6-phosphatase – is under substrate-level control by G-6-P, not allosteric control • Pyruvate carboxylase – Activated by acetyl-CoA – The fate of pyruvate depends on acetyl-CoA; pyruvate kinase (-), pyruvate dehydrogenase (-), and pyruvate carboxylase (+) • F-1,6-bisPase – is inhibited by AMP and Fructose-2,6-bisP – Activated by citrate - the reverse of glycolysis (w/o AMP) (w/ 25mM AMP) (F-2,6-BP) (F-2,6-BP) Figure 22.9 Inhibition of fructose-1,6-bisphosphatase by fructose-2,6bisphosphate in the (a) absence and (b) presence of 25 mM AMP. In (a) and (b), enzyme activity is plotted against substrate (fructose-1,6-bisphosphate) concentration. Concentrations of fructose-2,6-bisphosphate (in mM) are indicated above each curve. (c) The effect of AMP (0, 10, and 25 mM) on the inhibition of fructose-1,6-bisphosphatase by fructose-2,6-bisphosphate. Activity was measured in the presence of 10 mM fructose-1,6-bisphosphate. (AMP) • Fructose-2,6-bisP – is an allosteric inhibitor of F-1,6-bisPase – is an allosteric activator of PFK – synergistic effect with AMP • The cellular levels Fructose-2,6-bisP are controlled by phosphofructokinase-2 and fructose-2,6-bisPase which is bifunctional enzyme – F-6-P allosterically activates PFK-2 and inhibits F2,6-BisPase – Phosphorylation by cAMP-dependent protein kinase inhibits PFK-2 and activates F-2,6-bisPase Figure 22.10 Synthesis and degradation of fructose-2,6bisphosphate are catalyzed by the same bifunctional enzyme. 22.3 – How Are Glycogen and Starch Catabolized in Animals? A balanced diet provides carbohydrate each day, mostly in the form of starch. ∙ If too little carbohydrate is supplied by the diet, glycogen reserves in liver and muscle tissue can also be mobilized The starch and glycogen are digested by amylase ∙ ∙ ∙ -Amylase is an endoglycosidase -- (1→4) cleavage b-Amylase is an exoglycosidase (In plants) It cleaves dietary amylopectin or glycogen to maltose, maltotriose and other small oligosaccharides Figure 22.11 Hydrolysis of glycogen and starch by -amylase and bamylase. • -Amylase can cleave on either side of a branch point • But activity is reduced near the branch points and stops four residues from any branch point • limit dextrins • Debranching enzyme cleaves "limit dextrins" • Two activities of the debranching enzyme – Oligo(1,4→1,4)glucanotransferase – (1→6)glucosidase Figure 22.12 The reactions of glycogen debranching enzyme. Transfer of a group of three -(1 4)-linked glucose residues from a limit branch to another branch is followed by cleavage of the -(1 6) bond of the residue that remains at the branch point. Metabolism of Tissue Glycogen is Regulated Digestive breakdown is unregulated - nearly 100% • But tissue glycogen is an important energy reservoir - its breakdown is carefully controlled • Glycogen consists of "granules" of high MW range from 6 x 106 ~ 1600 x 106 • Glycogen phosphorylase cleaves glucose from the nonreducing ends of glycogen molecules • This is a phosphorolysis, not a hydrolysis • Metabolic advantage: product is a glucose-1-P; a potential glycolysis substrate Figure 22.13 The glycogen phosphorylase reaction. 22.4 – How Is Glycogen Synthesized? Glucose units are activated for transfer by formation of sugar nucleotides • What are other examples of "activated form"? – acetyl-CoA : acetate – Biotin and THF : one-carbon group – ATP : phosphate • Leloir showed that glycogen synthesis depends on sugar nucleotides – UDP-glucose pyrophosphorylase catalyzes the formation of UDP-glucose – ADP-glucose is for starch synthesis in plants Glucose-6-P Glucose-1-P + UTP → UDP-glucose + pyrophosphate. Figure 22.14 The UDP-glucose pyrophosphorylase reaction is a phosphoanhydride exchange, with a phosphoryl oxygen of glucose-1-P attacking the -phosphorus of UTP to form UDP-glucose and pyrophosphate. Glycogen Synthase Forms -(1 4) glycosidic bonds in glycogen • The glycogen polymer is built around aprotein Glycogenin (a protein core) – First glucose is linked to a tyrosine -OH on glycogenin – Sugar units can be added by the action of glycogen synthase • Glycogen synthase transfers glucosyl units from UDP-glucose to C-4 hydroxyl at a nonreducing end of a glycogen strand. (Fig. 22.15) Figure 22.15 The glycogen synthase reaction. Branching enzyme: • Glycogen branches are formed by amylo-(1,4→1,6)-transglycosylase, also called branching enzyme • -(1 6) linkages, which occurs every 8-12 residues • Transfer of 6- or 7-residue segment from the nonreducing end Figure 22.16 Formation of glycogen branches by the branching enzyme. Sixor seven-residue segments of a growing glycogen chain are transferred to the C-6 hydroxyl group of a glucose residue on the same or a nearby chain. 22.5 – How Is Glycogen Metabolism Controlled? A highly regulated process involving reciprocal control of glycogen phosphorylase and glycogen synthase • Activation of Glycogen phosphorylase (GP) is tightly linked to inhibition of glycogen synthase (GS), and vice versa • Regulation involves both allosteric control and covalent modification 22.5 – How Is Glycogen Metabolism Controlled? 1. Allosteric control • Glycogen phosphorylase allosterically activated by AMP and inhibited by ATP, glucose-6-P and caffeine • Glycogen synthase is stimulated by glucose-6-P Phosphorylation of GP and GS 2. Covalent modification • In chapter 15 (p496) showed that protein kinase converted phosphorylase b (-OH, inactivated) to phosphorylase a (-OP, activated) • Glycogen synthase also exists in two distinct forms – Active, dephosphorylated glycogen synthase I – Less active, phosphorylated glycogen synthase D (glucose-6-P dependent) Casein kinase Glycogen Synthase Kinase 3 (GSK 3) PP1: Phosphoprotein phosphatase 1 SPK: Synthase-phosphorylase kinase (phosphorylase kinase) • Glucagon and epinephrine activate adenylyl cyclase (chapter 15) – cAMP activates kinases and phosphatases that control the phosphorylation of GP and GS – stimulate glycogen breakdown • Dephosphorylation of both glycogen phosphorylase and glycogen synthase is carried out by phosphoprotein phosphatase-1 (PP1) – PP1 inactivates glycogen phosphorylase and activates glycogen synthase Hormones Regulate Glycogen Synthesis and Degradation Storage and utilization of tissue glycogen and other aspects of metabolism are regulated by hormones, including insulin, glucagon, epinephrine, and the glucocorticoids • Insulin is a response to increased blood glucose • Insulin triggers glycogen synthesis when blood glucose rises – Between meals, blood glucose is 70-90 mg/dL – Glucose rises to 150 mg/dL after a meal and then returns to normal within 2-3 hours Figure 22.17 Insulin triggers protein kinase cascades that stimulate glycogen synthesis. (glucose uptake, GLUT4) (F-1,6-BP & PEPCK) (PFK & PK) Figure 22.17 The metabolic effects of insulin. As described in Chapter 32, binding of insulin to membrane receptors stimulates the protein kinase activity of the receptor. Subsequent phosphorylation of target proteins modulates the effects indicated. Insulin • Insulin is secreted from the b-cells in the pancreas into the pancreatic vein, empties into the portal vein system (to liver) Figure 22.18 The portal vein system carries pancreatic secretions such as insulin and glucagon to the liver and then into the rest of the circulatory system. Hormonal Regulation Glucagon and epinephrine • Glucagon and epinephrine stimulate glycogen breakdown - opposite effect of insulin – Glucagon (29 AA-res) is also secreted from -cells in pancreas and acts in liver and adipose tissue only – Epinephrine (adrenaline) is released from adrenal glands and acts on liver and muscles • A cascade is initiated that activates glycogen phosphorylase and inhibits glycogen synthase Figure 22.20 Glucagon and epinephrine activate a cascade of reactions that stimulate glycogen breakdown and inhibit glycogen synthesis in live and muscles, respectively. PFK-2 isoforms in liver (ser32) and heart (ser466 & ser483) responds oppositely to PKA Hormonal Regulation • The phosphorylase cascade amplifies the signal – 10-10 to 10-8 M epinephine →10-6 M cAMP →Protein kinase (PK) →30 molecules of phosphorylase b kinase / PK →800 molecules of phosphorylase a →Catalyzes the formation of many molecules of glucose-1-P • The result of these actions is tightly coordinated stimulation of glycogen breakdown and inhibition of glycogen synthesis The difference between Epinephrine and Glucagon Both are glycogenolytic but for different reasons • Epinephrine is the fight or flight hormone – – – – Rapid breakdown of glycogen Inhibition of glycogen synthesis Stimulation of glycolysis Production of energy • Glucagon is for long-term maintenance of steadystate levels of glucose in the blood – activates glycogen breakdown – activates liver gluconeogenesis • Glucagon do not activate the phsphorylase cascade in muscle Cortisol and glucocorticoid • Glucocorticoids are steroid hormones that exert distinct effects on liver, skeletal muscle, and adipose tissue • Cortisol is a typical glucocoticoid • In skeletal muscle (catabolic) – promotes protein breakdown – decrease protein synthesis • In liver – stimulates gluconeogenesis – increases glycogen synthesis – Activates amino acid catabolism & urea cycle Figure 22.21 The effects of cortisol on carbohydrate and protein metabolism in the liver. 22.6 – Can Glucose Provide Electrons for Biosynthesis? Pentose Phosphate Pathway Hexose monophosphate shunt Phosphogluconate pathway 1. Provides NADPH for biosynthesis 2. Produces ribose-5-P for nucleotide synthesis • Several metabolites of the pentose phosphate pathway can also be shuttled into glycolysis • Operates mostly in cytoplasm of liver and adipose cells, but absent in muscle Pentose phosphate pathway • Begins with glucose-6-P, a six-carbon, and produces 3-, 4-, 5-, 6, and 7-carbon sugars, some of which may enter the glycolytic pathway • Two oxidative processes followed by five non-oxidative steps • NADPH is used in cytosol for reductive reaction-- fatty acid synthesis Figure 22.22 The pentose phosphate pathway. The numerals in the blue circles indicate the steps discussed in the text. Transketolase: 6 & 8 Transaldolase: 7 Begins with Two Oxidative Steps 1. Glucose-6-P Dehydrogenase – – – – Begins with the oxidation of glucose-6-P The products are a cyclic ester (the lactone of phosphogluconic acid) and NADPH Irreversible 1st step and highly regulated Inhibited by NADPH and acyl-CoA 2. Gluconolactonase – – – Gluconolactone hydrolyzed →6-phospho-Dgluconate Uncatalyzed reaction happens too Gluconolactonase accelerates this reaction Figure 22.23 The glucose-6phosphate dehydrogenase reaction is the committed step in the pentose phosphate pathway. Figure 22.24 The gluconolactonase reaction. 3. 6-Phosphogluconate Dehydrogenase – An oxidative decarboxylation of 6phosphogluconate – Yields ribulose-5-P and NADPH – Releases CO2 Figure 22.25 The 6-phosphogluconate dehydrogenase reaction. The Nonoxidative Steps • • • • Five steps, only 4 types of reaction... Phosphopentose isomerase – converts ketose to aldose Phosphopentose epimerase – epimerizes at C-3 Transketolase (TPP-dependent) – transfer of two-carbon units Transaldolase (Schiff base mechanism) – transfers a three-carbon unit • Phosphopentose isomerase – converts ketose to aldose – Ribose-5-P is utilized in the biosynthesis of coenzymes, nucleotides, and nucleic acids Glucose-6-P + 2 NADP+ + H2O → ribose-5-P + 2 NADPH + 2 H+ + CO2 Figure 22.26 The phosphopentose isomerase reaction involves an enediol intermediate. • Phosphopentose epimerase – An inversion at C-3 Figure 22.27 The phosphopentose epimerase reaction interconverts ribulose-5-P and xylulose-5-phosphate. The mechanism involves an enediol intermediate and occurs with inversion at C-3. • Transketolase (TPP-dependent) – transfer of two-carbon units – The donor molecule is a ketose and the recipient is an aldose Figure 22.28 The transketolase reaction of step 6 in the pentose phosphate pathway. Figure 22.29 The transketolase reaction of step 8 in the pentose phosphate pathway. Figure 22.30 The mechanism of the TPPdependent transketolase reaction. Ironically, the group transferred in the transketolase reaction might best be described as an aldol, whereas the transferred group in the transaldolase reaction is actually a ketol. Despite the irony, these names persist for historical reasons. • Transaldolase (Schiff base, imine) – transfers a three-carbon unit – Yields erythrose-4-P & Fructose-6-P Figure 22.31 The transaldolase reaction. Figure 22.32 The transaldolase mechanism involves attack on the substrate by an active-site lysine. Departure of erythrose4-P leaves the reactive enamine, which attacks the aldehyde carbon of glyceraldehyde-3-P. Schiff base hydrolysis yields the second product, fructose-6-P. Variations on the Pentose Phosphate Pathway • • • • 1) 2) 3) 4) Both ribose-5-P and NADPH are needed More ribose-5-P than NADPH is needed More NADPH than ribose-5-P is needed NADPH and ATP are needed, but ribose-5-P is not Figure 22.33 When biosynthetic demands dictate, the first four reactions of the pentose phosphate pathway predominate and the principal products are ribose-5-P and NADPH. 2) More Ribose-5-P than NADPH is needed by the cell. Synthesis of ribose-5-P can be accomplished without making NADPH, by bypassing the oxidative reactions of the pentose phosphate pathway 3) More NADPH than ribose-5-P is needed by the cell. This can be accomplished if ribose-5-P produced in the pentose phosphate pathway is recycled to produce glycolytic intermediates 4) Both NADPH and ATP are needed by the cell, but ribose-5-P is not. This can be done by recycling ribose-5-P, as in case 3 above, if fructose-6-P and glyceraldehyde-3-P made in this way proceed through glycolysis to produce ATP and pyruvate, and pyruvate continues through the TCA cycle to make more ATP Figure 21.25 The Calvin-Benson Cycle of reactions