C1 Revision
Food additives.
• Make food look and taste better, and last
longer.
• Anti-oxidants stop the food from reacting
with oxygen.
• Emulsifiers help oil and water mix.
– Hydrophobic and hydrophilic.
• The E means it has passed a safety test,
the number tells you what it does.
Cooking.
• Proteins denature.
– Heat changes their shape permanently.
• Starch grains burst.
• Cooking is an irreversible chemical
change.
• NaHCO3 → Na2CO3 + CO2 + H2O
Perfume.
• Acid + Alcohol → Ester and Water.
– Easily evaporates.
– Non toxic.
– Doesn't react with water.
– Doesn't irritate your skin.
– Insoluble in water.
Solutions.
• A solute dissolves in a solvent making a
solution.
• Its all about attraction.
– The solvent needs to be able to break the
bonds of the solute and make stronger bonds
with them.
What is oil made of???
•Oil is a Mixture of Hydrocarbons
•They are called:
• Alkanes and
• Alkenes
Chemicals
• Name
chemical formula
display formula
H
methane, CH4
H
C
H
H
3d shape.
An example of an alkane
• Methane
• All single bonds
• Saturated
an example of an alkene
• Ethene
• Contains a double bond
• unsaturated
Alkanes and alkenes.
• Alkanes:
– C-C
– ‘saturated‘
– CnH2n+2
– Can’t make
polymers.
– Very unreactive.
– Does nothing to
bromine water.
• Alkenes:
– C=C
– 'unsaturated'
– Cn H2n
– Used to make
polymers.
– Very reactive.
– Turns bromine
colourless.
Naming hydrocarbons
NO. OF CARBONS
NAME
1
Meth-
2
Eth-
3
Prop-
4
But-
5
Pent-
Fractional distillation.
• Hydrocarbon molecules contain only carbon and
hydrogen atoms.
• Crude oil can be separated by fractional
distillation,
– because they have different boiling and
condensation points.
• The crude oil is heated to vapourise it
(evaporated or boiled).
• The most volatile fraction, i.e. the molecules
with the lowest boiling points, boil or evaporate
off first and go to the top of the column.
How do we separate this
mixture??
• Fractional
Distillation
• As you go up the
column to the:
– Boiling point
decreases
– Intermolecular
forces decrease
– Volatility/ignition
increases
Hydrocarbon
Bonds.
• Down the pic above the molecule gets ...
• ... bigger as the carbon atom number in the molecule
increases.
• ... more viscous as the intermolecular forces between
molecules increases.
• ... higher melting point as more energy is needed to
overcome the intermolecular forces holding the
molecules together.
• ... higher boiling point as more energy is needed to
overcome the increasing intermolecular forces between
the liquid molecules.
• ... less flammable as they become less volatile, again
due to increasing intermolecular forces.
How do we make the fractions
more useful??
• What process is used to break long
carbon molecules (like tar) into
smaller, more useful molecules (like
petrol)?
• Cracking
• Need a catalyst
• High pressure
Cracking.
• In the catalytic cracker long chain molecules are
split apart or ‘cracked’.
• This is another example of thermal decomposition.
H H H H H H H H
H C C C C C C C C H
H H H H H H H H
hexane
Heat
pressure
H H H H H H
H C C C C C C H
Used as H H H H H H
Octane
catalyst
H
+
H
C C
a fuel
C8H18 C6H14 + C2H4
H
H
ethene
Ethene
is used
to make
plastics
Polymers.
• Monomers stick together and make polymers.
• Unsaturated monomers have a double bond between 2
carbons.
H H H H H H H H H H And
H
H
H C C C C C C C C C C lots
C C
more..
H
H
H H H H H H H H H H
1
2
3
4
5
thousands
This is called addition polymerisation and is written as:
H
n
H
C
C
ethene
H
H
Pressure
high
temperature
catalyst
H H poly(e)thene
C C
H H n
Making Polymers
• What do we start
with???
• An AlKENE
Making Polymers
• The DOUBLE bond is broken leaving:
Polypropene
• Ethene is only one alkene. Other unsaturated
molecules such as propene, vinyl chloride and
styrene can also be polymerised to produce a
range of plastics. E.g. propene
n
H
H
H
C C
C
H
propene
H
H
H CH3
Poly(propene)
C C
H H
n
• PVC
n
Cl
H
C C
H
H
Vinyl chloride
H Cl
C C
H H
pvc
n
Fuels.
• Oil is running out, and is none renewable.
• Oil slicks, acid rain, and green house
problems.
• Things that are important about choosing
fuels are:
– How
– How
– How
– How
– How
much energy it gives out.
much it costs.
easy it is to store.
poisonous it is.
much pollution it gives off.
Burning fuels.
• Burning hydrocarbons always gives off water
vapour.
• Enough oxygen allows complete combustion
giving off carbon dioxide as well.
• Too little oxygen gives off carbon monoxide
instead (and less oxygen).
• Cobalt blue goes pink for water
• Lime water goes cloudy for carbon dioxide.
Burning hydrocarbons
The apparatus below is used to test the
products of combustion of a hydrocarbon.
Suction
pump
icewater
Candle wax is the
hydrocarbon here
Lime water
Liquid collected
can be tested with
anhydrous cobalt chloride
paper (bluepink).
any hydrocarbon + oxygen water + carbon dioxide
Incomplete Combustion of Alkanes
• In the absence of an
adequate supply of air,
alkanes may react to form
carbon monoxide and water.
• Carbon monoxide is highly
poisonous and this is one
reason why gas boilers
must be serviced regularly.
A carbon
monoxide
detector
Methane + oxygen water
2CH4 + 3O2
+ carbon monoxide
4H2O
+
2CO
Energy.
• Exothermic
– Gives out energy
– More bonds are
made than broken
• Endothermic
– Takes in energy.
– More bonds are
broken than made.
It takes 4.2 Joules of energy to increase the temp of 1g of water by
1C
Energy transferred (J) = mass of water (g) x 4.2 x temp change (C)
Energy of fuel (J/g) = energy transferred (J) / mass of fuel burnt (g)
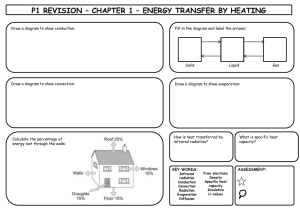
Energy for the Home
Module P1 Revision
• The thermal energy in a mass is the
total energy of all the particles in the
mass
• Thermal energy (heat) is measured
in joules (J)
• The temperature is a measure of
how hot something is (how fast the
average particle is moving)
• Temperature is measured in degrees
Celsius (°C)
Like water flowing downhill, from higher
places to lower places
• Thermal energy transfers from high
temperature places to low
temperature places
• The bigger the temperature
difference the quicker the heat
energy transfers
The Thermogram
• Temperature is
shown by the
colour
• Hot = red/yellow
• Cold = blue
Heating and Cooling
• To raise the temperature of an object
energy must be added (by heating
it)
• To lower the temperature of an
object energy must be taken away
(by cooling it)
Specific Heat Capacity, SHC
• The amount of energy added or
subtracted to change the
temperature of a substance depends
on:
• Its mass
• The material
• The temperature change
Specific Heat Capacity, SHC
• The Specific Heat Capacity of a
substance is the energy needed to
change the temperature 1 kg of the
substance by 1o
• The greater the SHC the more heat
energy it can store
Specific Latent Heat, SLH
• When a solid melts or a liquid boils it
requires energy, even though its
temperature does not change
• The energy is needed to break the
bonds between the molecules
Specific Latent Heat, SLH
• When a liquid solidifies or a gas
condenses energy is released even
though the temperature does not
change
• The energy is released as the atoms
or molecules join together and slow
down
Specific Latent Heat, SLH
• The Specific Latent Heat, SLH, is the
energy required or released by 1 kg
of a substance when it melts or
freezes, evaporates or condenses
Changes of state
90
80
Temperature
70
CONDENSING
BOILING
60
GAS
50
40
30
SOLIDIFYING
MELTING
LIQUID
20
SOLID
10
0
1
2
3
4
5
6
Time
7
8
9
10
11
12
Insulation
• Air is a good insulator,
(all gases are good insulators)
Any material that traps pockets of air
is a good insulating material
Eg: Expanded polystyrene, fibreglass
wool,
Double glazing, feathers, fur
Insulating
the house
• Loft insulation
• Cavity Wall
insulation
• Double glazing
• Carpets
• Draught
excluders
Payback time
• Payback time =
Original cost ÷
annual saving
Efficiency
• The more efficient a machine or device is,
the more of its INPUT energy is
transferred into a USEFUL energy OUTPUT
90 J Light Energy
USEFUL OUTPUT
100 J Electrical
Energy INPUT
10 J Heat Energy
WASTED OUTPUT
Efficiency = 90/100 = 0.9 = 90%
Payback time
• Money spent on insulating your home is
money well spent – but some methods are
better than others:
• Loft insulation may cost £500 to buy, and
save £250 each year in heating costs
• So it would take 2 years to save the
original cost: Payback time is 2 Years
Heat Energy Transfer (1)
• CONDUCTION:- Transfer of Thermal
Energy via particle to particle, atom
to atom
– Mainly occurs in solids
– “Hotter” Particles/atoms vibrate faster,
taking up more space so the solid
expands
– Best conductors are metals, due to free
electrons enabling rapid transfer of
energy
Heat Energy Transfer (2)
• CONVECTION:- transfer of heat energy
through liquids and gases
– “Hotter” particles/atoms move faster, so they
take up more space and the gas or liquid
expands and becomes less dense
– “Hotter,” less dense, masses of water or air will
rise
– “Colder”, more dense, masses of water or air
will sink
• CONVECTION CURRENTS will develop,
transferring energy everywhere else
Heat Energy Transfer (3)
• RADIATION:- EVERY object that is
HOTTER than its surroundings will
emit heat energy as INFRARED
RADIATION until it is the same
temperature as its surroundings
• This can be seen by infrared cameras
and sometimes felt by the skin
INFRARED (1)
• Black surfaces absorb infrared best
– Black cars get hot in the sun, white ones stay
cool
• Black surfaces emit infrared best
– Radiators should be black – but who wants black
radiators?
• White/silver surfaces reflect infrared
– NEWS FLASH! Space tourists travel to the sun behind a
giant mirror and survive!
• White/silver surfaces emit least
– So why is my radiator white?!
INFRARED (2)
• USED FOR:
– Burglar alarms: “heat sensors” detect
infrared
– Cooking: infrared heats the surface of
food, cooking it
– Control: Remote controls for TVs,
videos, DVDs
– Information: transfer of data to/from
computer mouse
MICROWAVES
• Have longer wavelength (lower frequency)
than infrared, so transfer less energy.
• Are reflected by metals, but go through
plastics and glass
• Used by mobile phones, satellites and
radar
• One particular wavelength used for
cooking because it is absorbed by water
molecules which then move more quickly
(ie they get “hotter”) – this then cooks the
food
ANALOGUE SIGNALS
• These are waves
that continuously
vary
• They can have
many different
values
• Any interference
can not be
removed: signal
quality can only
get worse
DIGITAL SIGNALS
• These are waves that only have two
values:
– High/Low
On/Off
1 or 0
• Interference or distortion can be
removed or is not recognised by the
receiver
• Signals can be boosted or amplified
without increasing interference
DIGITAL SIGNALS
Surface of DVD disc
Computer Binary Code
Digital signal
WAVES in general
Wavelength
Amplitude
Frequency = number of cycles (0scilations) per second (Hertz)
Speed of wave (m/s) = wavelength (m) x frequency (Hz)
Wave Characteristics
• There are two main types of wave
– Longitudinal, e.g. Sound, P-waves
– Transverse, e.g. Light, S-waves
Vibrational direction
Vibrational direction
Energy flow
Transverse Wave
Energy flow
LongitudinalWave
Wave Characteristics
• All waves can be
REFLECTED
The angle of reflection = the
angle of incidence
TOTAL INTERNAL
REFLECTION
Optical fibres can carry signals
without losing as much energy
Signals are more secure: more
difficult to listen in
Wave Characteristics
• All waves can be
REFRACTED
• Because light
SLOWS down in
more dense
materials like glass
Wave Characteristics
• All waves can be
DIFFRACTED
• When a gap or
obstacle is the same
width as the
wavelength the waves
spread out through it,
or around it
• When the wavelength
is smaller the
spreading is less
obvious
ELECTROMAGNETIC WAVES
• Electromagnetic waves are
transverse
• At the lowest energy, lowest
frequency and longest wavelength
are radiowaves
• Next come microwaves, infrared,
visible, ultraviolet, x-rays
• At the highest energy, highest
frequency and shortest wavelength
are gamma rays
LASERS
• Laser light consists of
lightwaves that are all
in phase with each
other
• This makes a tight
beam with great
intensity
• Can be used to cut
wood, steel and flesh
with great accuracy
• Reflects precisely off
CD and DVD discs
Wireless Technology
• Transmitter and receiver do not need to be physically
connected
• Available 24/7
• Radio waves can be reflected around the world through
the IONOSPHERE (a layer of ionised air high in the
atmosphere)
Wireless Technology
• Microwaves can be sent around the world by
being RECEIVED and RE-TRANSMITTED from
geostationary satellites
• Microwaves can be transmitted from phone
mast to phone mast if in line of sight
NOT
TO
SCALE
INTERFERENCE
• Different TV and radio channels use
different frequencies to broadcast
their programs
• If these frequencies are nearly the
same the receiving TV or radio will
get both programs – this is
INTERFERENCE
SEISMIC WAVES : P-WAVES
• P-Waves = Primary waves = “Pressure” waves
= longitudinal waves
• Are transmitted through solids, liquids and
gasses
• The denser the substance the quicker they
travel
• Sound travels at
– 330m/s in air
1500m/s in water
5000m/s in steel
SEISMIC WAVES : S-WAVES
• S-waves = Secondary waves = “sideways”
waves = transverse waves
• Only travel through solids or semi solid
material
• Travel more slowly through the Earth than PWaves
Structure of the Earth
This is what the interior looks like – we
think!
But
how
do we
know?
Structure of the Earth
• If the Earth was simply solid all the way
through
– Then the waves from an earthquake would be
felt everywhere around the world
STRUCTURE OF THE EARTH
What the S-Waves tell us
• Observation: The S-Waves are not detected
over nearly half of the Earth
• Explanation: The waves are blocked by a
Liquid Core
STRUCTURE OF THE EARTH
What P-Waves tell us
• Observation: There is a shadow zone where
P-Waves are not detected
• Explanation: A liquid core causes refraction
of the waves
•
•
•
•
•
•
ULTRAVIOLET
RADIATION
UV radiation can damage DNA in
skin cells
Small amounts cause tanning
Large amounts may cause skin
cancer
Dark skin absorbs some UV
reducing the amount reaching the
deeper layers of skin
The OZONE layer absorbs some
UV reducing the amount reaching
the Earth’s surface
CFCs reduce OZONE, CFCS have
been banned from current use
CLIMATE CHANGE
• The Earth’s climate changes naturally
as the sun changes
• Volcanic dust and ash and man-made
pollution can reflect sunlight away –
cooling the planet
• Greenhouse gases “trap” heat in –
warming the planet
• Greenhouse gases include CO2 ,
Methane and Nitrous Oxides (NOXs)
That’s all folks
• Remember
– Read the question at least twice, or until
you understand what it is asking you
– If the question asks you to “Describe...”
or “Explain…” then write a complete
sentence for each mark that can be
given
– Read through your answers to make
sure that they make sense
– Nonsense award Examiners marks
cannot for!
Biology
Unit 1
What’s the difference
between fitness and
health?
• Fitness is ability to do physical
activity
• Health is being free from
infection
How can we measure
fitness?
• Strength
• Stamina
• Speed
• Flexibility
• Agility
• Cardiovascular efficiency
• How many words can you
find that are to do with
fitness and exercise?
R
B
D
A
N
P
Q
B
S
Z
L
D
E
E
P
E
N
E
R
G
Y
A
O
A
S
S
T
R
T
V
W
H
C
X
J
O
P
L
M
O
N
V
A
T
Y
Q
C
U
I
S
W
B
P
C
I
G
A
U
L
L
R
V
T
I
D
C
E
D
L
S
A
R
A
D
D
C
A
N
B
G
E
F
K
L
T
U
D
C
U
A
N
A
E
R
O
B
I
C
I
V
U
D
X
V
S
R
B
D
O
D
A
J
B
R
E
A
T
H
E
K
N
Your beating heart gives
your blood pressure
• Blood pressure can be:
–Diastolic (When the heart
relaxes)
–Systolic (When the heart
contracts)
• What are the units for blood
pressure?
–mmHg
Blood pressure too
high?!
• Burst blood vessels
• Damage to brain
• Stroke
• Kidney damage
Blood pressure too
low?!
• Dizziness
• Fainting
• Poor circulation
•Blood pressure is
affected by age
and lifestyle (diet,
smoking,
alcohol…)
RESPIRATION
What’s the equation?
GLUCOSE
PLUS
OXYGEN
GIVES
CARBON
DIOXIDE
PLUS
WATER
PLUS
ENERGY
•Can you remember it?
•Try saying it to yourself!
Glucose + oxygen carbon dioxide + water +
(energy)
FATIGUE
•When we do hard exercise,
we respire
ANAEROBICALLY and
LACTIC ACID builds up in
our muscles.
ANAEROBIC
RESPIRATION
• Respiration without oxygen!
• Glucose lactic acid + energy
• MUCH LESS ENERGY RELEASED IN
ANAEROBIC RESPIRATION
BALANCED DIETS
• Vary depending on age, gender
and amount of activity
• Carbohydrate – The body’s energy
resource
• Fat – An insulator and an energy resource
• Protein – For growth and repair
• Vitamins – Keep the body functioning
• Minerals – Keep the body functioning
• Fibre – Keeps food moving along the gut
Can you remember the food
types and what they are
needed for?
• Carbohydrate – The body’s energy
resource
• Fat – An insulator and an energy resource
• Protein – For growth and repair
• Vitamins – Keep the body functioning
• Minerals – Keep the body functioning
• Fibre – Keeps food moving along the gut
CARBOHYDRATES
Are made from…
SIMPLE SUGARS (E.G. GLUCOSE)
FATS
• Are made from…
• FATTY ACIDS AND GLYCEROL
PROTEINS
• Are made from…
• AMINO ACIDS
• Animal proteins are known as ‘first
class’ proteins…
• …because they contain all the
essential amino acids the body
needs.
• Lack of protein causes
KWASHIORKOR
• BMI (body mass index) = mass
(g)
(height (m))2
• BMI can be used to help us
understand whether a person is
underweight, normal,
•A desire for perfection, low
self-esteem and poor self
image can lead to a poor
diet and increased risks of
poor health.
DIGESTION
•Chemical
Chemicaldigestion
digestionisis
the
the break
break down of large insoluble
down of large insoluble food
food molecules into smaller
molecules
into
smaller
more
more soluble ones by
soluble
ones
by
enzymes
for
enzymes for absorption into
absorption
the blood
the blood into
plasma
or lymph.
CAN YOU
MEMORISE
IT?
plasma or lymph.
•FOOD MOLECULES ARE
BROKEN DOWN BY
SPECIFIC ENZYMES.
CARBOHYDRATES are broken
down by CARBOHYDRASE
PROTEIN is broken down by
PROTEASE
FAT/LIPID is broken down by
LIPASE
Where does digestion
take place?
• The mouth
• The stomach
• The small intestine
• Stomach acid aids enzyme function.
• Small molecules are absorbed into
the blood in the small intestine by
DIFFUSION.
DISEASE
Diseases and disorders
can be caused by:
• Infectious microbes
• Genetic inheritance
• Vitamin deficiency
• Mineral deficiency
DEFICIENCIES
• No vitamin C Scurvy
• No iron Anaemia
BODY DISORDERS
• Inability to control blood sugar level
diabetes
GENETIC DISORDERS
• Red – green colour blindness
• Diseases like MALARIA are caused by
PARASITES.
• MALARIA is spread when the parasite
is carried from person to person by a
mosquito (the vector).
• How can knowledge of how vectors
spread disease help to control
infection
Immunity to disease
• Active:
– The body remembers a pathogen
invasion and white blood cells can
respond quickly next time it happens!
• Passive:
– Injecting a person with antibodies (e.g.
Protection from a snake bite)
The immune response
• The body’ white blood cells produce
antibody, specific to the pathogen
Can you order these
pictures?
2
4
5
1
3
THE EYE
Can you remember the labels?
REFLEXES ARE...
FAP
A
S
T
U
T
O
M
A
T
I
C
R
O
T
E
C
T
I
V
E
CANA
YOU
REMEMBER
THE LABELS?
What
is
this?
motor
neurone
away
• Sensory neurones carry impulses
________ from a sense organ.
• Motor neurones carry impulses to an
effector
_________
(muscle or gland)
DRUGS
What are the effects of the
following types of drug?
• Depressants
• Hallucinogens
• Painkillers
• Performance enhancers
• Stimulants
• Depressants – slow down brain function
• Hallucinogens- change what a person
sees/hears
• Painkillers – Stop nerve impulses so no
pain is felt
• Performance enhancers – develop muscles
• Stimulants – speed up brain function
The effects of drinking
alcohol
• Do silly things
• Easily lose balance
• Find it hard to talk
clearly
• Liver cirrhosis
• Drink driving
The effects of smoking
tobacco
• Nicotine is addictive
• Carbon monoxide
reduces oxygen
absorption
• Particulates collect
and block lungs
• Tar causes lung cancer
HOMEOSTASIS
• Keeping everything in balance
– Water
– Temperature
– Blood sugar
– Hormones
•TOO HOT –
HYPERTHERMIA
(NORMAL BODY TEMPERATURE IS 37 DEGREES CELCIUS)
•TOO COLD HYPOTHERMIA
Female sex
hormones,
progesterone
and oestrogen,
are produced in
ovaries
the ________
The pancreas
produces the
hormone
insulin which
_______,
controls blood
sugar level
The male sex
hormone,
testosterone, is
produced in the
testes
________
The genetic code is a set of
instructions that provide each
organism with its characteristics
DNA is a double helix
• It looks like a twisted ladder
• The rungs of the ladder are made up
of 4 bases.
Females have 2 ‘X’
chromosomes
Males have an X and a Y
chromosome
• Humans have 46 chromosomes (23
pairs)
• Human sex cells (gametes) contain
23 chromosomes
• When two sex cells meet 23 +23
= 46!
• THIS IS FERTILISATION
How do we get our
characteristics?
• Genes are inherited
• Humans show variation (we all have
differences)
• What causes variation?
Causes of variation
• Genes mixed up in gametes
• Genes coming from two different
parents (fertilisation)
• Mutations
– Radiation
– Chemicals (mustard gas)
Can you think of any
conditions that are inherited?
• Red – green colour blindness
• Cystic fibrosis
• Sickle cell anaemia
• Haemophilia
DOMINANT OR RECESSIVE?
• Dominant characteristics:
– A curved little finger
– Ability to roll your tongue
• Dominant characteristics tend to be
more common than recessive
characteristics.