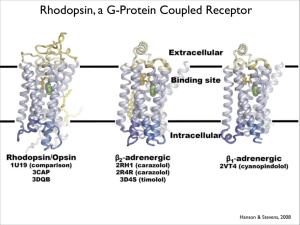
Rhodopsin
advertisement

Rhodopsin Christen Eberhart Rhodopsin Sequence The Eye •Rhodopsin is found in the rods that are located in the eye •Rods are composed of stacked disks •Rhodopsin is densely packed into each disk •Rods are responsible for black and white vision •Rhodopsin works best at dim light, responsible for night vision (too much light will saturate the protein) •Rod density is found greater on the outer edges of the retina (peripheral vision) Opsin & Retinal • Rhodopsin is made up of the protein opsin with the chromophore, retinal, covalently attached • The linkage occurs at Lys-296 Rhodopsin Structure • • • • • • 348 amino acids, 40 kD 7 transmembrane alpha helices connected by six loops of varying lengths 1 short alpha helix along the cytoplasmic membrane 2 antiparallel beta sheets at E-II and 2 antiparallel beta sheets at the NH2-terminal Cys-322 and Cys-323 attach to membrane by palmitic acid residues Functional monomer, however, can form dimers Retinal • Retinal is a derivative of Vitamin A, which is a derivative of betacarotene • Isomerization of cis-retinal to transretinal by light causes a conformation change in rhodopsin which triggers a signal • Rhodopsin absorbs at green-blue light (500 nm) which makes the protein appear reddish-purple Retinal Binding Site • • • • • • Lys-296 covalently attaches to retinal by a Schiff base Glu-113, Gly-114, Ala-117, Thr118, Gly-120, and Gly-121 side chains make a binding pocket for the retinal Thr-118, Tyr-268, and Ile-189 possibly could interact with the C9 methyl on the retinylidene group Met-207, His-211, Phe-212, Tyr268, and Ala-269 surround the Bionone ring of the retinal Tyr-43, Met-44, Leu-47, Thr-94 and Phe-293 region surrounds the Schiff base Ala-169 may interact with the Bionone ring in the activated state when retinal is trans Helical Wheel of the Short Alpha Helix • Hydrophobic side chains hold helix parallel to membrane • Hydrophilic side chains provide a key point of contact with the G-protein (Transducin) Stabilization between the Helices •Amino acids that provide stabilization of structure between five helices are shown •Disulfide bond between Cys110 and Cys-187 •Ala-299 peptide carbonyl hydrogen bonds with Asn-55 and Asp-83 •Asp-83 connects by a water molecule to Gly-120 •Asn-78 hydrogen bonds to Ser-127, Thr-160, and Trp161 •Asn-302 bonds through a water molecule with Asn-83 G-protein couple receptor (GPCR) • 7 transmembrane receptor that sense molecules outside the cell and activate inside signal transduction pathways and cellular responses (Rhodopsin) • Found only in eukaryotes • GPCR activates a G-protein (Transducin) by exchanging its GDP for GTP • GPCR is bound to a G-protein while in its inactive state • Once a GPCR is active, the G-protein detaches • G-protein is made up of 3 subunits (Gα, Gβ, and Gγ) • Once G-protein is activated, the Gα subunit activates another protein (phosphodiesterase) and detaches from the other two subunits Rhodopsin Transducin: Gα is red, Gβ is blue, and Gγ is yellow Phototransduction Pathway • • • • • • • Light activates Rhodopsin which activates Transducin by exchanging its GDP for GTP When active, Transducin’s alpha subunit dissociates from the other two subunits The active Transducin then activates a membrane bound protein called phosphodiesterase Phosphodiesterase hydrolyzes cGMP The hydrolyzes of cGMP, leads to the ion channel closing and the initiation of an action potential To return back to the inactive state Rhodopsin Kinase phosphorylates the cytosolic tail of rhodopsin which inhibits the activation of transducin Arrestin then binds to the phosphorylated rhodopsin further inhibiting activity Inactive to Active Form • Active (all trans form of rhodopsin) form still awaits a crystal structure • These pictures are based on computer simulations • Picture on the right: a is the non-active form, b-d is over time changing to the active from • Picture on the bottom shows a potential binding groove opening up in the active form on the cytoplasmic side Multiple Alignment Sequence Red-highly conserved important amino acid Blue-conserved important amino acid Purple-important amino acid Black-highly conserved amino acid Grey-conserved amino acid Retinitis Pigmentosa • Group of genetic eye conditions where the retina of the eye slowly and progressively degenerates • Night blindness occurs early on, then tunnel vision, and then many years later eventually blindness by the age of 40 • Up to 150 different point mutations can lead to RP, which causes misfolding of the protein • The mutant proteins then aggregate together and disrupt the visual phototransduction pathway • Rhodopsin mutations make up 25% cases of RP References • • • • • • • • Choi G, et al (2002) Structural studies of metarhodopsin II, the activated form of the G-protein coupled receptor, rhodopsin. Biochemistry, 41(23): 7318-1324. Berg JM, Tymoczko JL, Stryer L (2002) Biochemistry. New York: W.H. Freeman and Co. Krisha GA, et al (2002) Evidence that helix 8 of rhodopsin acts as a membrane-dependent conformational switch. Biochemistry, 41 (26): 82988309. Palczewski K, et al (2000) Crystal structure of rhodopsin: A G-protein coupled receptor. Science, Vol. 289, pp. 733-4. Terakita A, et al (2002) Functional interaction between bovine rhodopsin and G protein transducin. Journal of Biological Chemistry. 277 (1): 40-46. Helical Wheel (http://kael.net/helical.htm) Butterfly and Flower (photograph) (http://www.allaboutvision.com) Magnify (photograph) (http://www.flickr.com/photos/dfrighini/3240329545/)