How Cells Read the Genome: From DNA to Protein
advertisement

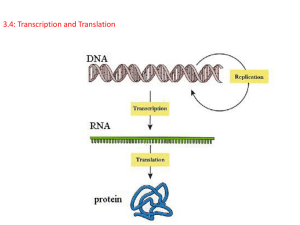
How Cells Read the Genome: From DNA to Protein Chapter 6 How Cells Read the Genome ► Transcription ► Translation ► Folding of Proteins ► Evolution of the “Central Dogma” How Cells Read the Genome The Genomes of Multicellular Organisms: a State of Disarray! ► ► ► ► Introns Irregularities in Gene Density Little organization relating to gene function Adjacent genes often show no relatedness How the Cells Read the Genome The “Central Dogma” DNA RNA Protein Variations in the Central Dogma RNA Splicing RNA as the final gene product Transcription RNA vs DNA ► ► ► ► Ribose as opposed to deoxribose sugar Single stranded Uracil in place of Thymine RNA molecules w/ structural and catalytic properties Transcription General Features of Transcription ► ► ► ► ► Produces RNA using one strand of DNA as template molecule Only small portion of DNA is transcribed Begins w/unwinding of sm portion of DNA exposing bases Transcript elongated by complementary base pairing by RNA polymerase RNA transcript shorter than DNA Transcription RNA Polymerase ► ► ► ► ► Transcribes DNA by catalyzing the formation phosphodiester bond btwn nucleotides Moves along DNA unwinding DNA just ahead of active site Extends chain in the 5’ to 3’ direction Hydrolysis of high-energy bonds provides energy Many copies of RNA from same gene in small amount of time Transcription RNA Polymerase vs DNA Polymerse ► RNA Polymerase catalyzes addition of ribonucleotides ► Can begin RNA synthesis without primer ► Not as accurate as DNA Polymerse; RNA Polymerase 1 error/104 nucleotides vs 1 error/107 nucleotides ► Both have proof reading capability Transcription Different Types of RNA ► mRNA; RNA copied from genes that ultimately direct synthesis of proteins (35% total RNA) ► Final product of minority of genes is RNA itself: tRNA, rRNA, snRNA (majority of total RNA) ► Tens of thousands of different mRNA transcripts; 10-15 copies of ea species/cell Transcription in Procaryotes Start and Stop Signals embedded in DNA ► ► ► ► ► ► ► ► Promoter= DNA sequence RNA Polymerase binds to initiate RNA synthesis RNA Polymerase’s sigma subunit (bact) binds to promoter & opens helix One of exposed strands serves as template After synthsis of ~10 bases sigma subunit of RNA Polymerase disassociates RNA Polymerase undergoes structural changes moving forward rapidly synthesizing RNA at ~50 bp/sec RNA Polymerase continues until termination signal of string of A-T preceded by “hairpin loop” Termination signal causes RNA Pol to disassociates from RNA releasing DNA Free RNA Polym complexes once again w/ sigma subunit Transcription in Procaryotes Transcription Transcription Promoter and Terminator Signals ► Heterogeneous but contain related consensus sequence recognized by sigma subunit of RNA Pol ► Precise promoter sequence governs affinity for RNA Pol = “strength” ► Can be predicted by algorithms but need to be independently verified ► Promoters are assymetric; RNA Pol can bind in one orientation and extend in 5’ to 3’ direction only ► Terminators more heterogeneous than promoters; ability of RNA to fold into “hairpin loop” is most important feature Transcription in Eucaryotes 3 Types of RNA Polymerases of similar in structure in Eucryotes RNA Pol I- transcribes tRNA, rRNA, smRNAs ► RNA Pol II- transcribes genes encoding proteins ► RNA Pol III- transcribes tRNA, rRNA, smRNAs ► Transcription Transcription in Eukaryotes vs Procaryotes ► ► Nucleosomes and higher order chromatin packaging RNA Pol requires General Transcription Factors Transcription General Transcription Factors (Eukaryotes) ► ► ► ► Assemble at promoters of all RNA Pol II transcripts Facilitate binding of RNA Pol II Aid in opening DNA strands for transcription to begin Release RNA Pol from promoter into elongation mode once transcription has begun Transcription 5 different General Transcription Factors Transcription Other Proteins Required by RNA Pol in Eucaryotes 1. 2. 3. 4. Transcriptional activators- bind to specific sequences and facilitate binding of GTFs and RNA Pol Mediators- enable activators to interact w/ GTFs and RNA Pol Chromatin Remodeling Complexes- allow greater accessibility to DNA Many proteins required to initiate transcription, > 100 subunits Transcription Elongation Elongation factors= ensures that RNA Pol does not disassociate before end of gene; assoc. w/ RNA Pol shortly after initiation of transcription ► DNA topoisomerases removes superhelical tension ► DNA gyrases uses ATP to pump supercoils into DNA ► Elongation tightly coupled to RNA processing ► Transcription RNA Processing 1. 2. 3. Eucaryotic mRNA capped at 5’ end polyadenylated at 3’ end Introns removed Phosphorylation of RNA tail CTD ► ► ► consists of domain of repeated 52 times of 7 aa containing 2 serines that are phosphorylated phosphorylation of tail promotes disassoc of RNA Pol from proteins presents at start of transcription allows new set of proteins that function in elongation and pre-mRNA processeing, to assoc Transcription Capping of Pre-mRNAs Capping of 5’ end w/ modified guanine nucleotide occurs after ~25 bases synthesized 3 enzymes involved in capping process 1. phosphatase removes one P’ from 5’ end of RNA 2. guanyl transferase adds GMP to 5’ end 3. methyl transferase adds methyl grp to guanosine ► Capping enzymes bind to phosphorylated tail of RNA Pol ► Cap binds CBC (cap binding complex) that facilitates RNA processing and export ► ► Transcription General Features of RNA Splicing ► ► ► ► ► Involves two transesterification reactions to join exons and remove intron in form of lariat 5 additional RNAs, > 50 proteins, and lots of ATP required Complexity ensures accuracy Alternative splicing occurs in 60% of human genes Increase coding potential and facilitates evol of new protein sequences Transcription Sequences Mark Where Splicing Occurs Intron size varies from 10-100,000 nucleotides ► 3 conserved nucleotide sequences 5’ splice site 3’ splice site branch points that forms base of excised lariat ► Transcription Spliceosome Mediates Splicing of RNA performed primarily by 5 snRNA molec (U1, U2, U4, U5) forming spliceosome core ► snRNA molec recognize intron-exon borders and participate in splicing chemistry ► Spliceosome complex of RNA and protein ► More than 50 proteins invovled ► Transcription Mechanism of RNA Slicing 1. spliceosome recognizes splicing signals on pre-mRNA, brings ends of intron together 2. branch point site recognized by BBP and U2AF 3. U2 snRNP displaces BBP base pairing w/ branch point consensus seq 4. U1 snRNP base pairs w/ 5’ splice site junction 5. U4/U6•U5 triple snRNP enters 6. RNA-RNA rearrangements disrupts U4/U6 base pairing to enable U6 to displace U1 at 5’ splice junction; U4 exits 7. U2 and U6 snRNPs in spliceosome form 3d RNA structure bringing 5’ junction into position near branch chain A for first esterification Transcription Mechanism of RNA Slicing 8. 5’ and 3’ junctions brought together via U5 snRNP for second esterification 9. snRNPs remain bound to lariat while splice product released Transcription Spliceosome and ATP Hydrolysis ► ► ► ► ► Not required for splicing chemistry Needed for assembly and rearrangements RNA helicase requiring ATP needed to break RNA-RNA interactions All steps except assoc of BBP w/ branch chain A, and U2 w/ 5’ splice site require ATP and other proteins Removal of snRNP from lariat requires RNA-RNA interactions that are dependent on ATP hydrolysis Transcription Assembly of Spliceosome ► ► ► ► ► Occurs as pre-mRNA emerges from transcribing RNA Pol Components of the spliceosome are carried on RNA Pol tail and transferred to nascent pre-mRNA Exon size tend to be uniform ~150 bp Spliceosome assembly occurs co-transcriptionally, splicing sometimes occurs posttranscriptionally Spliceosome proteins SR (rich in Ser and Arg) assemble on exon and mark off 3’ and 5’ site starting at 5’ end of mRNA, assembly occurs in conjunction w/ U1 snRNA and U2AF Transcription Plasticity of RNA Splicing ► ► ► ► Splicing mech selected for flexibility Flexibility enables cell to regulate pattern of RNA splicing Alternative splicing when diff proteins can be made from same gene Splicing patters regulated so diff forms of protein produced at diff times and in diff tissues Transcription Self-Splicing Introns ► ► ► ► Group I Intron= reactive G nucleotide attacks the initial phosphdiester bound cleaved during the spicing rxn Group II Intron= reactive A in intron seq is attaching grp, and lariat intermediate generated Sequence of self=splicing introns is critical; RNA folds in specific 3d conformation that brings 5’ and 3’ junctions together and provides precisiely positioned reactive grps to perform chemistry Pre-mRNA splicing mech evolved from Grp II Splicing spliceosomal snRNPs took over structural and chemical roles of Grp II Introns so sequence constraints no longer needed Transcription Group I Introns Group II Introns Transcription Processing of 3’ End of pre-mRNA Termination signs are transcribed into RNA and recognized proteins as RNA Pol transcribes thru them ► CstF (cleavage stimulating factor F) and CPSF (cleavage and processing specificity factor) proteins assoc w/ RNA Pol tail transferred to RNA as it emerges ► Transcription Processing the 3’ End of the Pre-mRNA ► Additional proteins assemble w/ CstF and CPSF to perform processing: 1. RNA is cleaved 2. Poly-A-Polymerase adds ~200 A’s to 3’ end of cleaved product 3. RNA Pol II continues to transcribe after premRNA has been cleaved; several 100 bases before falls off template and transcription terminates 4. RNA downstream of cleavage is degraded Transcription Selective Export of Mature mRNAs from Nucleus How does cell distinguish btwn rate mature mRNA and debris from mRNA processing? ► Export highly selected and coupled to correct mRNA processing ► mRNA exported only if appr set of proteins are bound including: 1) cap binding complex 2) snRNP proteins absent 3) proteins that mark complete splicing Selective Export of Mature mRNAs from Nucleus Transcription hnRNPs= heterogeneous nuclear ribonuclear proteins, most abundant proteins that assemble on pre-mRNA as it emerges from RNA Pol ► Some hnRNPs remove hairpin helices from RNA so that splicing and other signals on RNA can be read ► hnRNPs excluded from exons, remain on excised introns marking them for nuclear retention and/or destruction ► Some reamin bound to fully processed mRNA and accompany them to cytoplasm Transcription Most of the RNA in the Cell Performs a Catalytic or Structural Function ► ~80% of total RNA is rRNA ► 3-5% of total RNA mRNA ► rRNA transcribed by RNA Pol I (which has no C-terminal tail) ► rRNA is neither capped or polyadenylated ► RNA components of ribosome are final gene products; growing cell syn ~10 million of ea type of rRNA ea cell generation Transcription rRNA Genes ► ► ► Mammalian cells contain 10 million ribosomes Multi-copy genes E. coli has 7 copies of its rRNA Humans ~200 copies on 5 chromosomes Xenopus ~600 copies single cluster on 1 chromosome 4 types of eucaryotic rRNAs ea present in one copy/ribosome 18S, 5.8S and 28S encoded by single lg precursor RNA-chemically modified 5S rRNA syn from separate cluster by Pol III- not chemically modified Transcription Chemical Modification of Lg rRNA Precursor ► 100 methylations of 2’-OH ► 100 isomerizations of uridines ► Function of chem. modification unknwn but may facilitate folding or assembly ► snoRNAs= small nucleolar RNAs guide in chemical modification and cleavage of precursor rRNA ► snoRNAs encoded in introns, esp those of ribosomal proteins, function in nucleolus Transcription Nucleolus as a Ribosome Producing Factory site for processing rRNAs and assembly into ribosomes ► lg aggregate of macromolecules including: rRNA genes, precursor rRNAs, mature rRNAs, rRNA processing enzymes, snoRNPs, ribosomal protein subunits, and some partially assembled ribosomes ► Size varies and reflects number of ribosomes cell is making; may occupy 25% of nuclear vol ► Also site where other RNAs produced and other RNA-protein complexes assembled (tRNAs, snoRNAs, U6 snRNP, telomerase) ► Translation From RNA to Protein ► ► ► ► Genetic code dictates how mRNA translated into aa sequence of protein Nucleotides read consecutively in grps of 3 (condons) Degenerate genetic code; 64 codons specify 20 aa 6 possible reading frames Translation tRNA as an Adaptor Molecule Recognizes and binds to codon and appr. aa ► ~80 nucleotdies ► Folds into clover leaf and then into L-shaped structure ► Two regions of unpaired nucelotdies at ends of L shaped molecule essential to function 1. anticodon= set of 3 consecutive nucleotides pairs w/complementary codon on mRNA 2. short ss region near 3’end where aa attaches ► Translation Redundancy/Degeneracy of Genetic Code More than one tRNA for many of the aa ► Some tRNAs base pair w/ more than one aa; Wobble Position ► Translation tRNAs ► ► ► ► ► ► Number and kinds of tRNAs varies across species ie: humans have 497 tRNA genes w/ only 48 diff anticodons represented Transcribed by RNA Pol III Some transcripts are spliced via cut-and-paste mechanism catalyzed by proteins (no lariat) occurs when tRNA is properly folded in cloverleaf conf Extensive modifications > 50 different kinds 1 modification/10 nucleotides Modifications essential to: 1) accuracy of tRNA attaching to correct aa 2) recognition of mRNA codon by tRNA anticodon Translation Aminoacyl-tRNA Synthetases Couple aa to appr set of tRNAs ► Aa specific ► Enzymatic reaction attaches aa to 3’ end of tRNA requires ATP ► High energy bond produced btwn aa and tRNA later used to covalently link aa to growing polypeptide chain ► Translation Editing by tRNA synthetases ►Selects correct aa in two step process: 1. correct aa highest affinity for active site 2. aa forced into second pocket whose dimensions exclude correct aa ►Those that enter second editing site are hydrolyzed from AMP- hydrolytic editing ►Raises overall accuracy of tRNA charging to 1 mistake/40,000 couplings ►Synthetase must also recognize correct tRNAs Translation Ribosome Structure: rRNA molecules + 50 different proteins Translation Ribosome Structure and Function Large and Small Subunits; rRNA sequence highly conserved ► 66% RNA; 33% protein ► rRNA responsible for: structure positioning tRNAs on mRNA catalytic activity in forming peptide bond ► Translation mRNA Decoded on Ribosomes ► ► ► ► ► Ribosomal subunits assemble on mRNA near 5’ end Ribosome translates mRNA in aa sequence using tRNAs as adaptors to add aa in correct sequence to end of growing polypeptide chain aa linked by peptide bond formation 2 aa added/sec eucaryotic cell; 20 aa/sec added by bact 4 impt ribosomal binding sites Translation Major Steps in Translation 1. Ribosomal Assembly and Initaition 2. Elongation 3. Termination and Release of Nascent Polypeptide Translation Ribosomal Assembly 1. 2. 3. 4. 5. Initiation tRNA-Met & eIFs bind sm rRNA subunit Sm rRNA subunit binds to 5’ end of mRNA recognizing CAP and 2 eIFs (eucaryotes) Sm rRNA scans for AUG start eIFs dissociate and lg rRNA binds Initiator tRNA-met now in P-site leaving A-site vacant for incoming aminoacyl rRNA to start protein synthesis Translation Procaryotes have Shine Delgarno Sequence Specific sequence few nucleotides upstream of AUG start ► AGGAGGU ► Binding site positions sm rRNA subunit at AUG start ► Bacterial mRNAs can be poly-cistronic; eucaryotes monocistronic ► Translation Elongation 1. 2. 3. tRNA carrying next aa in chain binds to ribo A-site base pairing w/ mRNA codon Peptide Bond Formation= carboxyl end released from tRNA at P-site and joined to free amino grp of aa linked to tRNA at A site forming new peptide bond Translocation= conformational chgs move mRNA 3 nucleotides along ribo resetting ribo for next acyl tRNA Translation Two Impt Elongation Factors Drive Translation ► ► EF-Tu Mechanism- resp. 99% accuracy a. Chged tRNA enters ribo bound to GTP form b. Codon recognition triggers GTP hydrolysis c. EF-Tu dissociates from ribo w/out tRNA d. Introduces 2 short delays btwn codonanticodon pairing and chain elongation EF-G binds in or near A site hydrolyzes GTP whose energy accelerates movement of bound tRNAs in A/P and P/E hybrid states Translation Termination ► ► ► ► ► Stop codons: UAA, UAG, UGA Stop codons recognized by “release factor” proteins Release factors cause peptidyl transferase to catalyze addition of water to peptidyl tRNA Hydrolysis frees COOH end of polypeptide chain from attachment to tRNA Ribosomal dissassembly Translation Proteins are Made on Polyribosomes Syn of avg protein varies 20 sec to 2-3 min ► mRNAs translated in form of polyribosomes w/ ribos spaced > 80 nucleotides ► Transcription and translation occur simultaneously in procaryotes ► Translation Quality Control of Translation ► ► ► ► Accuracy ~1 mistake/104 aa Speed of translation 20 aa incorporated/sec in pro. vs 2 aa/sec euk. Protein synthesis consumes more energy than any other biosynthetic process; 4 ATP equivalents/ aa Quality control mech to ensure mRNA is complete recognition of Cap and poly A tail by initiation complex Translation Antibiotics ►Inhibitors of bacterial protein synthesis are effective antibiotics ►Specificity of antibiotics useful for molecular cell biology studies Life and Death of Proteins Protein Maturation Protein folding begins while protein is being synthesized ► Maturation: unique 3d structure bind sm molecules modifications assemble w/ other proteins ► Life and Death of Proteins Protein Folding ► Steps in protein folding: 1. molten globule 2. slow phase ► Most of folding complete by time released from ribosome ► Molecular chaperones held guide folding of many proteins Life and Death of Proteins Molecular Chaperonins ► ► ► ► ► Two major families: hsp60 & hsp70 Affinity for exposed hydrophobic patches Massage protein into folded conformation via ATP hydrolysis Hsp70 acts early in life of protein; binds to stretch of 7 hydrophobic aa before protein leaves ribo Hsp60 acts later in proteins life, forms barrel into which proteins fed Life and Death of Proteins Proteosome ► Protein disposal apparatus dispersed throughout cytosolo ► Hollow cylinder of proteases form stack of 4 heptameric rings ► Ends composed of protein complex of ~20 polypeptides, 6 of which hydrolyze ATP to unfold proteins and move them into proteosome ► Acts on proteins marked by ubiquitin Life and Death of Proteins Ubiquitin conjugating system Multiubiquitin chain on target proteins recognized by receptors on proteosome ► Targeted proteins: misfolded, oxidized, or other abnormal aa ► Linear series of ubiquitin conjugates linked via lysine on target protein ► Life and Death of Proteins Regulated Destruction ► ► ► Activation of compments in proteolytic pathway E1, E2, E3 Degradation signals created in response to intracellular or extracellular signals N-terminal rule 12 destabilizing aa including Arg, Lys, His, Leu, Asp Try, Tyr, Asp, Glu, Asn, Gln