Agro 2013
advertisement

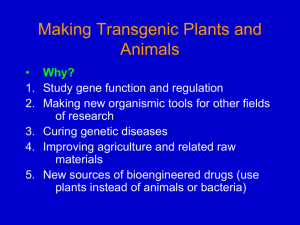
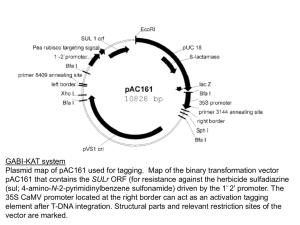
Plant Genetic Transformation All stable transformation methods consist of three steps: • Delivery of DNA into a single plant cell. • Integration of the DNA into the plant cell genome. • Conversion of the transformed cell into a whole plant. Agrobacterium-mediated Transformation Only known natural example of DNA transport between Kingdoms 1. (Virulent) strains of A. tumefaciens contain a 200-kb tumor inducing (Ti) plasmid 2. Bacteria transfer a portion of the plasmid DNA into the plant host (T-DNA). T-DNA •Infects at root crown or just below the soil line. •Can survive independent of plant host in the soil. •Infects plants through breaks or wounds. •Common disease of woody shrubs, herbaceous plants, dicots. •Galls are spherical wart-like structures similar to tumors. The T-DNA is transferred from the Bacteria into the Nucleus of the Plant 1. Stably integrates (randomly) into the plant genome. 2. Expression of genes in wild-type T-DNA results in dramatic physiological changes to the plant cell. 3. Synthesis of plant growth hormones (auxins and cytokinins) neoplastic growth (tumor formation) Genes required to breakdown opines for use as a nutrient source are harbored on the Ti plasmid in addition to vir genes essential for the excision and transport of the T-DNA to the wounded plant cell. T-DNA 23 kb tra vir genes for transfer to the plant pTi ~200 kb bacterial conjugation opine catabolism Agrobacterium chromosomal DNA pscA chvA chvB T-DNA-inserts into plant genome for transfer to the plant vir genes pTi tra bacterial conjugation opine catabolism oriV Ti Plasmid Ti Plasmid Agrobacterium can be used to transfer DNA into plants Ti plasmids and the bacterial chromosome act in concert to transform the plant 1. Agrobacterium tumefaciens chromosomal genes: chvA, chvB, pscA required for initial binding of the bacterium to the plant cell and code for polysaccharide on bacterial cell surface. 2. Virulence region (vir) carried on pTi, but not in the transferred region (T-DNA). Genes code for proteins that prepare the T-DNA and the bacterium for transfer. 3. T-DNA encodes genes for opine synthesis and for tumor production. 4. occ (opine catabolism) genes carried on the pTi allow the bacterium to utilize opines as nutrient. Generation of the T-strand Left Border Right Border T-DNA overdrive 5’ virD/virC VirD nicks the lower strand (T-strand) at the right border sequence and binds to the 5’ end. Generation of the T-strand Left border T-DNA Right border gap filled in virE virD/virC T-strand D 1. Helicases unwind the T-strand which is then coated by the virE protein. 2. ~one T-strand produced per cell. Left border T-DNA Right border D T-strand coated with virE virD nicks at Left Border sequence 1. Transfer to plant cell. 2. Second strand synthesis 3. Integration into plant chromosome Overview of the Infection Process pTi-based vectors for plant transformation: 1. Shuttle vector is a small E. coli plasmid using for cloning the foreign gene and transferring to Agrobacterium. 2. Early shuttle vectors integrated into the TDNA; still produced tumors. Shuttle plasmid E. coli conjugation pTi Agrobacterium Transformation of Arabidopsis plants Detergent added to allow bacteria to infiltrate the floral meristem. Dip floral buds in 1 ml of Agrobacterium culture for 5 to 15 min. Transformation of Arabidopsis plants 700 to 900 seeds per plant. Germinate on kanamycin plates to select transformants. 10 to 20 transformed plants per plant. 10 day old seedlings MiniTi T-DNA based vector for plants Disarmed vectors: do not produce tumors; can be used to regenerate normal plants containing the foreign gene. 1. Binary vector: the vir genes required for mobilization and transfer to the plant reside on a modified pTi. 2. consists of the right and left border sequences, a selectable marker (kanomycin resistance) and a polylinker miniTi for insertion of a foreign gene. MiniTi T-DNA based vector for plants a binary vector system T-DNA deleted kanr polylinker LB 1 RB ori bom modified Ti plasmid vir miniTi bom = basis of mobilization oriV 2 Alternate Methods of Transforming Plants: Particle Bombardment One way of physically introducing DNA into cells is with a particlegun. •Very tiny DNA-coated metal particles are suspended in a drop on a macroprojectile. •A discharge (from a gunpowder explosion or from breakage of a membrane enclosing a pressurized chamber) impels the macroprojectile. •The macroprojectile is stopped by a stopping plate, but the microprojectiles continue into the tissue below. •The DNA introduced with the particles is expressed Particle Bombardment using the Gene Gun 1. DNA- or RNA-coated gold/tungsten particles are loaded into the gun and you pull the trigger.