How Cells Harvest Energy
advertisement

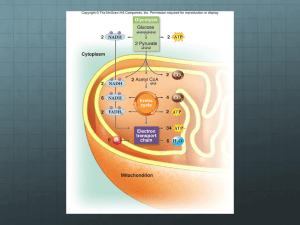
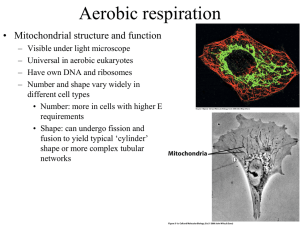
How Cells Harvest Chemical Energy ATP Is Universal Energy Source Photosynthesizers get energy from the sun Consumers get energy from plants or other organisms ENERGY is ALWAYS converted to the chemical bond energy of ATP Photosynthesis and Respiration are LINKED Photosynthesis Overall reaction: 6 CO2 + 12 H2O C6 H12O6 + 6 O2 + 6 H2O Often shown as 6 CO2 + 6 H2O C6 H12O6 + 6 O2 Photosynthesis Overview Sunlight 12 H2O 6 CO2 ATP Light Dependent Reactions ADP + P NADPH Light-Independent Reactions NADP+ 6 O2 Reactions occur in grana of the thylakoid membrane system Glucose-P + 6 H2O Reactions occur in stroma Overview of Cellular Respiration Glucose + 6 O2 6 CO2 + 6 H2O 30 - 32 ATP The overall reaction is exergonic. The energy given off is used to make ATP. O2 Breathing CO2 Lungs CO2 Bloodstream O2 Muscle cells carrying out Cellular Respiration Glucose 6 O2 6 CO2 6 H2O 30-32 ATP •Breathing and cellular respiration are closely related: Cellular Respiration and ATP Cellular respiration releases the energy stored in glucose in a series of steps The energy released is stored as ATP and released as heat Aerobic respiration is ~34% efficient Meaning… 34% of the energy stored in the glucose is captured and stored as ATP How Cells Make ATP ATP is made in two ways during cellular respiration: 1. Substrate level phosphorylation Glycolysis Citric acid cycle (Krebs cycle) 2. Oxidative phosphorylation Electrons transport system and chemiosmosis Substrate-level phosphorylation ENZYME ATP is made in an enzyme in a coupled reaction. Substrate gives energy and phosphate group (Pi) to ADP and makes ATP. Substrate Product Fig. 9.7 Oxidative phosphorylation Electron carriers (NADH and FADH2) deliver electrons to the Electron Transport Chain (ETC) on the inner membrane of the mitochondria Electrons are passed from membrane protein to protein. • Each transfer releases energy and pumps H+ out of the matrix Energy is used to create a chemical gradient • Gradient is used to drive ATP synthesis by the enzyme ATP synthase Overview of Cellular Respiration Carbohydrate Metabolism The first pathway of carbohydrate metabolism is called glycolysis. Glucose is the starting material for glycolysis. Glycolysis reactions occur in the cytoplasm. Glycolysis Step #1: Glucose is converted to glucose6-phosphate in a phosphorylation reaction Reaction is endergonic Reaction requires an input of ATP Step #2: A rearrangement reaction occurs to make fructose-6-phosphate Glycolysis Step #3: Another phosphorylation reaction occurs to made fructose-1,6-diphosphate Reaction is endergonic Reaction requires an input of ATP Step #4: Fructose-1,6-diphosphate is broken in to 2 three carbon compounds Glycolysis 5 more steps occur and 2 pyruvate are made These steps release energy and electrons. • Energy released is used to make ATP by substrate level phosphorylation • Electrons are attached to the electron carrier NAD+ to form 2 NADH The NADH deliver electrons and H+ to the electron transport system More on NADH Synthesis of NADH (simplified version): NAD+ + 2 e + H+ NADH Is NAD+ oxidized or reduced in this reaction? Glycolysis Energy requiring steps: 2 ATP invested Energy releasing steps: 2 NADH formed 4 ATP formed Net yield is 2 ATP and 2 NADH Does NOT require O2 Occurs in the cytoplasm Glycolysis Summary Where it occurs: First substrate: (starting “material”) End product: Also made: net gain of ____ ATP (why net?, how made?) _____ NADH (made from?) Glycolysis Summary Where it occurs: Cytoplasm First Substrate: Glucose (6C) End product: 2 Pyruvate (3C) Glycolysis Summary Also made net gain of 2 ATP made by substratelevel phosphorylation • Pathway requires an input of 2 ATP to start and makes a total of 4 ATP 2 NADH – each made from NAD+ ,2e and H+ Energy Releasing Pathways • What happens to the products of glycolysis depends upon cell conditions. Aerobic conditions • Preparatory step and Citric Acid/Krebs cycle • Electron transport chain Anaerobic conditions • Fermentation GLYCOLYSIS OR ANAEROBIC Conditions No oxygen present Net gain of 2 ATP AEROBIC RESPIRATION Oxygen present Net gain of 30-32 ATP GLYCOLYSIS OR AEROBIC RESPIRATION ANAEROBIC • Fermentation occurs • Type depends upon cell type • Reactions occur in cytoplasm • • • • Preparatory step Krebs Cycle Electron Transport Chain Reactions occur in mitochondria Pathways of Aerobic Respiration Glycolysis 1. followed by Pyruvate oxidation Citric Acid cycle 2. 3. Also called Krebs Cycle Electron Transport Chain (ETC) and Chemiosmosis Aerobic Conditions The first reaction that occurs after glycolysis is pyruvate oxidation Also called the Preparatory Step This reaction occurs as the pyruvate enter the matrix of the mitochondria Pyruvate Oxidation As the pyruvate enter the mitochondria each has a carbon removed and coenzyme A added Produced in the Prep. Step • 2 NADH (go to ETC) • 2 CO2 (diffuse out of mitochondria and cell) Pyruvate Oxidation Cytoplasm ------------------------------ Matrix of the mitochondria ------------------------------ Aerobic Respiration For each glucose metabolized the Preparatory Step makes • 2 NADH - go to ETC • 2 CO2 - diffuse out of mitochondria and cell • 2 Acetyl Co-A - enter into Citric acid cycle *aka – Krebs cycle Pyruvate Oxidation Summary Where and when it occurs: Substrate: End Product: Also made: ___________ ___________ Pyruvate Oxidation Summary Where and when it occurs: Occurs as pyruvate enter mitochondria, occurs under aerobic conditions Substrate: 2 Pyruvate (3C) End Product: 2 Acetyl-CoA (2C) Also made: 2 CO2 2 NADH Citric Acid Cycle = Krebs Cycle Step 1: Each Acetyl-CoA (2C) joins with an oxaloacetate (4C) to form a citrate (6C) Rest of the citric acid cycle reactions occur • Last reaction produces another oxaloacetate (4C) which joins with the next available acetyl-co A……. • ATP, NADH, FADH2, and CO2 are made in these reactions….see board CoA Acetyl CoA CoA 2 carbons enter cycle Oxaloacetate Citrate NADH H CO2 NAD CITRIC ACID CYCLE leaves cycle NAD Malate NADH ADP FADH2 P ATP Alpha-ketoglutarate FAD CO2 Succinate NADH H NAD leaves cycle H In the Krebs cycle, the metabolism of 2 pyruvates made from a single glucose produces: 2 ATP - by substrate-level phosphorylation 6 NADH - go to ETC 2 FADH2 - go to ETC 4 CO2 - diffuse out of mitochondria and cell Citric Acid Cycle Summary Where it occurs: Starting substrates: Last product of pathway: Also made (in total for 2 acetyl-CoA entering) ____ ____ ____ ____ CO2 ATP (method made by?) NADH FADH2 Citric Acid Cycle Summary Where it occurs: matrix of mitochondria Starting substrates: acetyl-CoA, oxaloacetate Last product of pathway: oxaloacetate Also made (in total for 2 acetyl-CoA) 4 CO2 2 ATP (by substrate level phophorylation) 6 NADH 2 FADH2 Electron Transport Chain (ETC) ETC occurs at electron carriers (proteins) located on the inner membrane of the mitochondria Electrons from NADH and FADH2 are passed from one electron carrier to the next. • Transfers are called red-ox reactions • Each transfer releases energy ETC Some of the electron carriers are also proton (H+) pumps • Use the energy released by the red-ox reactions (e transfer reactions) to pump H+ out of the matrix. H H H Protein complex Intermembrane space Inner mitochondrial membrane Mitochondrial matrix H Electron carrier H H ATP synthase FAD NAD NADH H H FADH2 Electron flow H H 1 2 O2 2 H H H Electron Transport Chain ADP H2O P ATP H Chemiosmosis ETC NADH and FADH2 each transfer 2e and H+ to a specific ETS protein Notice -- they do NOT start with the same ETC protein In the process are the NADH and FADH2 oxidized or reduced? H H H Protein complex Intermembrane space Inner mitochondrial membrane H Electron carrier H 1 2 O2 ATP synthase 2 H H Mitochondrial matrix H H FAD NAD NADH H H FADH2 Electron flow H H Electron Transport Chain H2O ADP P ATP H Chemiosmosis ETC H+ from the matrix follow the electrons into proton pumps At each proton pump the H+ are pumped out of the matrix into the intermembrane space This creates an electrical & chemical gradient • Form of _________ energy ETC Electron transfers stop when the last ETC protein transfers the 2e to oxygen which: Joins with H+ to form water The last electron acceptor is oxygen and water forms. (know this) Chemiosmosis and ATP Synthesis ….back to the H+ ions pumped into the intermembrane space The potential energy of H+ gradient is drive ATP synthesis at the enzyme ATP synthase ETC and ATP Synthesis The enzyme ATP synthase is embedded in the inner membrane of the mitochondria flow of H+ through this enzyme releases energy and this energy is used to make ATP . The Chemiosmosis and ATP Synthesis This method of making ATP is called Oxidative phosphorylation Also referred to as chemiosmosis Chemiosmosis and ATP Synthesis The more H+ pumped out of the matrix The steeper the gradient the more potential energy the more ATP that can be made by ATP synthase ETC and ATP Synthesis Each NADH made in the mitochondria results in enough H+ being pumped out of the matrix to make 2.5 ATP. FADH2 results in enough H+ being pumped out of the matrix to make 1.5 ATP. Each NADH from Glycolysis The NADH made in glycolysis must enter the matrix in order to deliver their electrons to the ETC How they “enter” the mitochondria depends upon the cell type. NADH from Glycolysis In most cells the 2 NADH made in glycolysis pass their electrons and H+ to FAD in the matrix making: 2 FADH2 -- take the electrons and H+ to the ETC where a total of ____ ATP are made NADH from Glycolysis In liver, heart, and kidney cells the 2 NADH made in the cytoplasm pass their electrons and H+ to NAD+ in the matrix making: 2 NADH -- which take the electrons and H+ to the ETC where a total of ____ ATP are made or 2 NADH ATP Synthesis Summary Glycolysis ____ ATP (net) (method?) ____ NADH ____ ATP (most cells) Preparatory step ____ ATP _____ NADH ____ ATP (method?) ATP Synthesis Summary Krebs Cycle ____ ATP (method?) ____ NADH ____ ATP (method?) ____ FADH2 ____ ATP (method?) NADH and FADH2 Summary Glycolysis 2 NADH • Made in the cytoplasm • How they enter the mitochondria depends upon the type of cell Preparatory Step 2 NADH Kreb’s Cycle 6 NADH and 2 FADH2 Fermentation Under anaerobic conditions the products of glycolysis enter fermentation reactions. All fermentation reactions occur in the cytoplasm. Fermentation The purpose of all types of fermentation is to regenerate NAD+ so that glycolysis can continue. Fermentation Cell’s have a limited supply of NAD + Under aerobic conditions the cell’s major source of NAD+ is the first step of the ETC Under anaerobic conditions the Krebs cycle and ETC stop • As a result NAD+ are no longer made in the mitochondria. Fermentation The two most common forms of fermentation are: Lacate fermentation Alcoholic fermentation Which type of fermentation occurs depends upon the organism. Lactate Fermentation Lactate fermentation occurs in: Humans and all other animals Many bacteria Lactate Fermentation 2 Pyruvate* (3C) 2 NADH 2 NAD+ reused in glycolysis 2 Lactate* (3C) * Also called: pyruvic acid and lactic acid Lactate build up in the cell results in: Increased blood supply to the area, which: Blood brings oxygen Blood “washes” out the lactate Lactate is taken to the liver where it is converted back to pyruvate (called the Cori cycle) • Too much lactate in the blood can cause acidosis Muscle cramps if the lactate levels get too high occurs - painful Alcoholic Fermentation Alcoholic fermentation occurs in: Yeast (a fungus) • used in making alcoholic beverages and “yeast” breads Many bacteria • Including those used to make Swiss cheese Alcoholic Fermentation 2 Pyruvate (3C) __________ 2 Acetaldehyde (2C) 2 NADH 2 NAD+ - reused in glycolysis 2 Ethanol (2C) Alcoholic Fermentation Ethanol (alcohol) builds up in the cell When it reaches too high a level it denatures the cell’s proteins. This results in cell death! Wild yeasts die at 4% alcohol, wine making yeasts die at 14% alcohol. Alcoholic Fermentation Reactions cannot be reversed. Remember, the lactate fermentation reaction is reversible • Lactate can be converted back to pyruvate in the liver, not in the cell it’s made in This is the end of the slides needed. The slides that follow are slides that give an overview of concepts related to the ETC. Electron Transfers and Energy Electron transfer reactions are called oxidation reduction reactions Oxidation – loss of electron(s) Reduction – gain of electron(s) H+ often follow the electrons Electron Transfers and Energy ALL cells use the transfer of electrons and H+ to capture some of the energy stored in chemical bonds Energy is temporarily stored in NADH and FADH2 • The stored energy is then used to make ATP Electron Transfers and Energy NAD+ FAD + H + + 2 e NADH + 2 H+ + 2 e FADH2 Is the NAD+ oxidized or reduced in this reaction? In general the reduced molecule is of greater energy due to the added energy of the electrons Electron Transfer Chains In mitochondria and chloroplasts there are electron transfer chains embedded in the inner membranes The passage of the electrons from electron transfer protein to protein results in creation of an electrochemical gradient This gradient is a form of stored energy and can be used to make ATP Electron Transfer Chains In mitochondria: NADH and FADH2 give electrons and H+ to specific proteins on the inner membrane of the mitochondria • this releases their stored energy H + follow the electrons into the proteins Electron Transfer Chains Energy given off by the electron transfers is used to pump H+ across the inner membrane into the outer compartment This creates a chemical/electrical gradient • A form of potential energy • An ATP-synthesizing enzyme uses this energy to make ATP