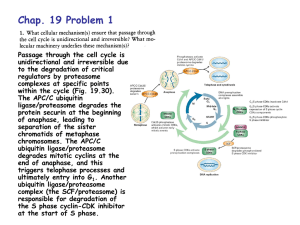
Cell cycle control by ubiquitylation
advertisement

The Inner Life of the Cell http://www.youtube.com/watch?v=2-p-QajenM0&feature=related Structural study of cell-cycle control proteins Current Opinion in Structural Biology 2002, 12:822–830 : Structural basis of ubiquitylation NATURE Reviews Cancer 2006, 6:369-381 :Ubiquitin ligases: cell-cycle control and cancer The control of the cell cycle Anti-proliferative signals Growth factor (mitogen) (CDK activating kinase) Cell cycle control by ubiquitylation (Structural study of SCF and APC) Ubiquitin The three dimensional structure of ubiquitin: contains 76 amino acids Simplified view of the cell-cycle control system Levels of cyclin expression during cell division are periodic1. This is the result of a constant synthetic rate coupled with a defined window in the cycle of specific proteolysis, which is executed by the ubiquitinproteasome system (UPS). Cell cycle control of SCF ubiquitin ligase by proteolysis of Cdk inhibitor protein P27 (CIP) CKIs, negative-regulators of cyclin–CDK kinase complexes, are also targeted for degradation by the UPS. Three-layer regulation of the cell cycle Therefore, the cell cycle is predominantly regulated by two types of posttranslational protein modification — phosphorylation and ubiquitylation. Overview of the ubiquitin-proteasome pathway Ubiquitin ligase (E3) enzyme complex A. Ubiquitin-protein ligases (also known as E3s) act at the last step of a three-enzyme cascade involving the ubiquitin-activating (E1) and ubiquitin-conjugating (E2) enzymes. B. The E3 mediates the transfer of ubiquitin from the E2 to the substrate protein by promoting the formation of an isopeptide bond between the Ub carboxy-terminus and specific lysine side chains on the substrate. C. E3s bind both the protein target and a cognate E2 and have a central role in conferring specificity to the ubiquitination pathway. D. The mechanism by which they promote ubiquitination has not been well understood. Two distinct types of E3s HECT-type E3s catalyse ubiquitination RING-type E3s do not appear to form by first forming an E3–ubiquitin thioester such an intermediate. They are intermediate. characterized by the presence of a RING zinc finger domain that binds the E2. The SCF (Skp1–Cullin–F-box protein) complexes A. The SCF complexes are RING-type E3s B. The largest family of ubiquitin–protein ligases. C. ubiquitinate a broad range of proteins involved in cell cycle progression, signal transduction and transcription. D. Deregulation of SCF-dependent proteolysis can contribute to neoplastic transformation. Human SCF complexes with demonstrated E3 activity SCFSkp2 : Cdk-inhibitor p27Kip1 SCFFbw7 : cyclinE SCFb-TrCP : b-catenin and IkB The composition of SCF complexes The SCF complexes are RING-type E3s that consist of A. Cul1 (776 residues), invariable B. Rbx1 (108 residues), C. Skp1 (163 residues) and variable D. F-box protein family (430 to.1,000 residues). Rbx1, which contains the RING domain, and Cul1 form a catalytic core complex that recruits a cognate E2 F-box proteins are characterized by an amino-terminal 40-residue F-box motif that binds Skp1 followed by protein–protein interaction modules such as leucine rich repeats or WD-40 repeats that bind substrate. How is it possible to ubiqutinate various substrate? E3 components in the UPS are thought to be primarily responsible for the specific recognition of a large number of target proteins. This requires both specificity and versatility, which are provided by the existence of 500–1,000 different E3 ligases. How is it possible to make various SCFs to ubiqutinate various substrate? A. The large number of F-box proteins in eukaryotic genomes (at least 38 in human) allows for the specific ubiquitination of a large number of functionally and structurally diverse substrates B. In addition to multiple F-box proteins, most higher eukaryotes also contain multiple homologues of the other SCF subunits, including two Rbx1 and five cullin family members (paralogues) conserved from C. elegans to humans. The schematic structures of SCF Cell-cycle regulation by the SCF complex and APC/C APC SCF Functions of the SKP1–CUL1–F-box-protein (SCF) complex Cell-cycle regulation by the SCF complex and APC/C The structure of Skp1 and Skp2 complex Overall structure of the SCFskp2 The N-terminal domain of Cullin1 N-terminal tip of repeat 1 that is the Skp1-F boxSkp2 binding site The C-terminal domain of Cullin1 and Rbx1 30 A° -wide groove Intermolecular b-sheet formed by Rbx1 and Cul1 C-terminal domain The Cul1 residues that contact Rbx1 are shown in light green, and the Rbx1 residues in pink. The zinc-finger (RING) domain of Rbx1 Rigidity of Cul1 scaffold required for SCF function To start investigating the importance of the rigid architecture of the Cul1 scaffold, we sought to construct a Cul1 mutant where the NTD and CTD interface is disrupted, and where the two domains are linked by a flexible linker (Fig. 5a). The SCFSkp2 complex with the wild-type (WT) Cul1 (lane 1) but not the linker mutant Cul1 (lane 3) The Cul1 linker mutant retains the ability to bind phosphorylated p27, in a manner dependent on the presence of Skp1, Skp2 and Cks1. promotes the Cks1-dependent polyubiquitination (Ubn ) of p27 in an in vitro ubiquitination assay reconstituted with purified components. Model of the SCFSkp2–E2 complex The principal stages of mitosis in human cells and chromosome segregation 1 2 3 4 5 6 1 : prophase, 2 : pro-metaphase 3 : metaphase 4, 5, 6 : early, mid, and late anaphase, respectively Fixed HeLa cells were stained for DNA (blue), microtubules (green) and kinetochores (red) Regulation of mitosis by ubiqutin ligase APC (anaphase promoting complex) Isolation of Native Human APC APC was immunoprecipitated from extracts of HeLa cells using CDC27 peptide antibodies. Bound complexes were subsequently eluted in their native form with an excess of antigenic peptide. (cullin-like) The peptide was subsequently separated from the eluted protein by gel filtration chromatography SDS–PAGE and silver staining analysis of the resulting fractions revealed all known 11 subunits of human APC whose identity was confirmed by immunoblotting (not shown) (RING-finger domain) Characterization of Native Human APC In the presence of purified ubiquitin, E1 and E2 enzymes, and ATP, the APC fractions were able to ubiquitinate a radiolabeled fragment of cyclin B in a dose-dependent manner Native electrophoreses of APC Electron Microscopy of Negatively Stained APC Diameter of 15 nm 3D Model of the APC Obtained by Cryo-Electron Microscopy Purified APC samples were imaged using liquid nitrogen temperature electron microscopy. About 13,000 molecular images of randomly orientated APC particles were interactively collected from digitized micrographs. A first set of characteristic APC views was obtained by multivariate statistical analysis and automatic classification. After angular reconstitution, a preliminary low resolution 3D structure was derived. Subsequently, the resolution of the structure was reiteratively improved by generating large number of reference images and performing multiple cycles of multireference alignment, automatic classification, and angular reconstitution. Using this procedure, a 3D model of the APC with a final resolution of 24 A° was generated. 140A° X 140A° x135A° in size