Lecture 8 - Transcription 4
advertisement

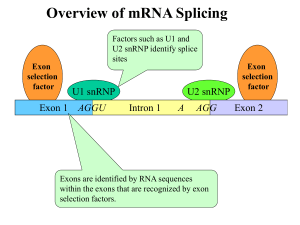
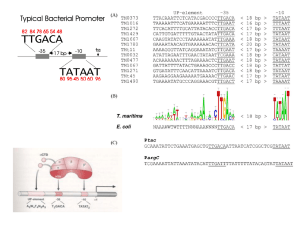
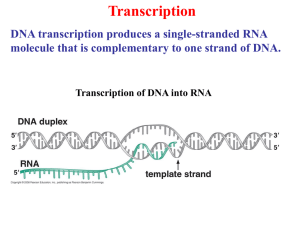
Biochemistry 201 Biological Regulatory Mechanisms January 30, 2012 Transcription in Eukaryotes REFERENCES Books: Chapter 17 of Molecular Biology of the Gene 6th Edition (2008) by Watson, JD, Baker, TA, Bell, SP, Gann, A, Levine, M, Losick, R. 589-632. Articles: Chromosome conformation capture (CCC) technologies de Wit, E. and de Laat, W. (2012) A decade of 3C technologies: insights into nuclear organization. Genes Dev. 26: 11-24. Elongation BBA2013-- Issue 1874 devoted to reviews of transcription elongation General Transcription Factors Matsui, T., Segall, J., Weil, P.A., and Roeder, R.G. (1980) Multiple factors required for accurate initiation of transcription by purified RNA polymerase II. J Biol Chem 255: 11992-11996. Thomas, M.C., & Chiang, C.M. (2006). The general transcription machinery and general cofactors. Critical reviews in Biochemistry & Molecular Biology, 41(3), 105-78. Muller, F, Demeny, MA, & Tora, L. (2007). New problems in RNA polymerase II transcription initiation: matching the diversity of core promoters with a variety of promoter recognition factors. The Journal of Biological Chemistry, 282(20), 14685-9. Mediator and Other Components *Kornberg, R.D. (2005) Mediator and the mechanism of transcriptional activation. Trends in Biochemical Sciences 30:235-239. Fan, X, Chou, DM, & Struhl, K. (2006). Activator-specific recruitment of Mediator in vivo. Nature Structural & Molecular Biology, 13(2), 117-20. Sikorski TW and Buratowski. (2009). The basal initiation machinery: Beyond the general transcription factors. Current Opinion in Cell Biology. 21 344-351. What Do Activators Do? Cosma, MP, Tanaka, T, & Nasmyth, K. (1999). Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter. Cell, 97(3), 299-311. Bryant, GO, & Ptashne, M. (2003). Independent recruitment in vivo by Gal4 of two complexes required for transcription. Molecular Cell, 11(5), 1301-9. Bhaumik, S.R., Raha, T. Aiello, D.P., and Green, M.R. (2004) In vivo target of a transcriptional activator revealed by fluorescence resonance energy transfer. Genes Dev 18: 333-343. Vakoc, CR, Letting, DL, Gheldof, ... Blobel, GA (2005) Proximity among Distant Regulatory Elements at the B–Globin Locus Requires GATA-1 and FOG-1. Molecular Cell 17:453-462 Fishburn, J., Mohibullah, N. and Hahn, S. (2005) Function of a eukaryotic transcription activator during the transcription cycle. Molecular Cell 18:369-378. Bulger M and Groudine M. Functional and Mechanistic Diversity of Distal Transcription Enhancers (2011). Cell 144:327-39 Role of the RNA Pol II CTD *McCracken, S, Fong, N, Yankulov, K, et al. (1997). The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature, 385(6614), 357-61. Tietjen,J. ……Ansari, A. Chemical-genomic dissection of the CTD code (2010) NMSB: 17: 1154-1162 Mayer, A. ….Cramer, P. Uniform transitions of the general Pol II transcription apparatus (2010) NMSB 17:1272-79 Buratowski, S (2009) progression through the RNA polymerase II CTD cycle ( Review). Mol Cell 36: 541-546 Chapman, R… Eick, D. Molecular evolution of the RNA polymerase CTD. Trends in Genetics (2008): Jun;24(6):289-96. Epub 2008 May 9. Review.PMID: 18472177 ChIP-exo technology Rhee, HS and BF Pugh (2011) Comprehensive genome wide Protein-DNA Interactions at single nucleotide resolution. Cell 147:1408 Rhee, HS and BF Pugh (2012) Genome-wide structure and organization of eukaryotic pre-initiation complexes Nature 483: 295 Elongation Control Rougvie A and Lis JT (1988) The RNA Polymerase II Molecule at the 5’ end of the uninduced hsp70 gene of D. melangaster is transcriptionally engaged. Cell 54: 795-804 Zobeck, KL….Lis Jt (2010) Recruitment timing and dynamics of transcription factors at the Hsp70 Loci in Living Cells Mol Cell 40 965-75 Peterlin, BM and Price DH (2006) Controlling the Elongation Phase of transcription with P-TEFb Mol Cell 23: 297 – 305 Nechaev S…..Adelman K. (2010) Global Analysis of short RNAs reveals widespread Promoter Proximal Stalling and Arrest of Pol II in Drosophila Science 327: 335-38 Gilchrist, DA,……Adelman, K. (2010) Pausing of RNA Polymerase II Disrupts DNA specified Nucleosome Organization to enable precise gene regulation. Cell 143: 540-51 Chen, Y.,….Handa, H.(2009) DSIF, the Paf1 complex and Tat-SF1 have nonredundant, cooperative roles in RNA polymerase II elongation. Genees Dev 23: 2765 -77 Liu, Y……Hahn, S. (2009) Phosphorylation of the transcription elongation factor Spt5 by yeast Bur1 kinase stimulates recruitment of the PAF complex. MCB 29: 4852-63 Wu, C-H,….Gilmour, D. ( 2003)NELF and DSIF cause promoter proximal pausing on the Hsp70 promoter in drosophila. Genes Dev 17: 1402-14 Kim, J Guemah M and Roeder, RG.(2010) The human PAD1 Complex Acts in Chromatin Transcription elongation both independently and cooperatively with SII (TFIIS) Cell 140: 491 -503 A link between Transcription and Translation Harel-Sharvit, L., …..and Choder M. (2010). RNA polymerase II subunits Link transcription and mRNA decay to translation. Cell 143: 552-63 Important Points 1. Transcription initiation at Pol II promoters on naked DNA templates in vitro requires the general transcription factors in addition to RNA polymerase II. 2. In vivo, transcription initiation also requires activators – proteins that bind directly to enhancers – as well as Mediator and enzymes that modify chromatin structure. 3. At a typical eukaryotic promoter, activators guide the assembly of Mediator, the general transcription factors, RNA polymerase and chromatin-modifying enzymes, often through weak, relatively non-specific interactions. There appears to be no set order of assembly from one promoter to the next. Moreover, different promoters have different requirements for these components. 4. The RNA polymerase CTD is a long series of 7-amino acid repeats. When transcription is initiated, serine 5 of the repeat is phosphorylated by TFIIH. As elongation proceeds, serine 5 is gradually dephosphorylated and serine 2 is gradually phosphorylated by enzymes carried along with the RNA polymerase. This dynamic pattern of modification couples transcription to processing of the newlysynthesized RNA. Eukaryotic Cells have three RNA polymerases TYPE OF POLYMERASE GENES TRANSCRIBED RNA polymerase I 5.85, 18S, and 28S rRNA genes RNA polymerase II all protein-coding genes, plus snoRNA genes, miRNA genes, siRNA genes, and some snRNA genes RNA polymerase III tRNA genes, 5S rRNA genes, some snRNA genes and genes for other small RNAs The rRNAs are named according to their “S” values, which refer to their rate of sedimentation in an ultra-centrifuge. The larger the S value, the larger the rRNA. Transcription Initiation by PolII requires many General Transcription Factors RNA Pol II + NTPs + DNA containing a real promoter NO TRANSCRIPTION promoter RNA Pol II + NTPs nuclear extract + DNA with real promoter TRANSCRIPTION INITIATION and ELONGATION Purification scheme for partially purified general transcription factors. Fractionation of HeLa nuclear extract (Panel A) and nuclear pellet (Panel B) by column chromatography and the molar concentrations of KCl used for elutions are indicated in the flow chart, except for the Phenyl Superose column where the molar concentrations of ammonium sulfate are shown. A thick horizontal (Panel A) or vertical (Panel B) line indicates that step elutions are used for protein fractionation, whereas a slant line represents a linear gradient used for fractionation. The purification scheme for pol II, starting from sonication of the nuclear pellet, followed by ammonium sulfate (AS) precipitation is shown in Panel B. (Figures are adapted from Flores et al., 1992 and from Ge et al., 1996) NAME # OF SUBUNITS FUNCTION TFIIA 3 Antirepressor; stabilizes TBP-TATA complex; coactivator TFIIB 1 Recognizes BRE;Start site selection; stabilize TBP-TATA; pol II/TFIIF recruitment 1 ~10 Binds TATA box; higher eukaryotes have multiple TBPs Recognizes additional DNA sequences; Regulates TBP binding; Coactivator; Ubiquitin-activating/conjugating activity; Histone acetyltransferase; multiple TAFs TFIID TBP TAFs TFIIF 2 Binds pol II; facilitates pol II promoter recruitment and escape; Recruits TFIIE and TFIIH; enhances efficiency of pol II elongation TFIIE 2 Recruits TFIIH; Facilitates forming initiation-competent pol II; promoter clearance TFIIH 9 ATPase/kinase activity. Helicase: unwinds DNA at transcription startsite; kinase phosphorylates ser5 of RNA polymerase CTD; helps release RNAP from promoter Transcription Initiation by RNA Pol II The stepwise assembly of the Pol II preinitiation complex is shown here. Once assembled at the promoter, Pol II leaves the preinitiation complex upon addition of the nucleotide precursors required for RNA synthesis and after phosphorylation of serine resides within the enzyme’s “tail”. The Pol II promoter has many recognition regions Positions of various DNA elements relative to the transcription start site (indicated by the arrow above the DNA). These elements are: BRE (TFIIB recognition element); there is also a second BRE site downstream of TATA TATA (TATA Box); Inr (initiator element); DPE (downstream promoter element); DCE (downstream core element). MTE (motif ten element; not shown) is located just upstream of the DPE. The GTFs are not sufficient to mediate activation: Discovery and isolation of Mediator from Yeast GTFs and RNA Pol II Tx 1 unit VP 16 GAL4 1 unit crude lysate 10 units 4 years mediator 50 units Is Mediator Required for Transcription of all Pol II -transcribed genes? Control: ts subunit of Pol II Rpb1 Rpb1ts high T Compare levels of all mRNAs using microarrays (37˚C for 45 min) mRNAs decrease over time according to their half-lives. A few mRNAs remain at some level (stable mRNAs). Subunit of Mediator Srb4ts Same basic pattern Subunit of TF II H Kin28ts Same basic pattern Mediator is very large and has diverse roles A) PIC model from EM-study of polII (brown)-TFIIF (light blue) and X-ray structure of TBP (white) -IIB (yellow)--DNA; Arrow indicates direction of trx B) Model for PIC-mediator was produced by superimposing an EM structure of Mediator-PolII on the PIC in A; head, middle and tail regions shown The Head region interacts with PolII-TFIIF complex; the mutants with general effects on Trx are located in this region; the tail region interacts with activators; mutants have more specific effects on transcription SAGA is another important complex with multiple roles in transcription, including being a coactivator The core of SAGA, containing the Taf substructure (Yellow), is surrounded by three domains responsible for distinct functions: activator binding (Tra-1), histone acetylation Gcn5), and TBP regulation (Spt3). This structural organization illustrates an underlying principle of modularity that may be extended to our understanding of other multifunctional transcription complexes. The TAFs in TFIID also serve as coactivators Assembly of PIC in presence of mediator, activators and chromatin remodelers Genomic Level Snapshots ChIP-exo, a new technology for determining location at the genome scale Comparison of ChIP-exo to ChIP-chip and ChIP-seq for Reb1 at specific loci. The gray, green, and magenta filled plots, respectively show the distribution of raw signals, measured by ChIP-chip using Affymetrix microarrays having 5 bp probe spacing (Venters and Pugh, 2009), ChIP-seq, and ChIP-exo. Aggregated raw Reb1 signal distribution around all 791 instances of TTACCCG in the yeast genome Many Paths to the PIC Buratowski, 2009 The factors and assembly pathways used to form transcriptionally competent preinitiation complexes can be promoter dependent. (1) TBP assembling onto promoter regions via TFIID leads to recruitment of the other basal initiation factors, as outlined in the stepwise assembly pathway. In S. cerevisiae, this pathway is most often utilized at TATA-less genes. At some mammalian promoters, histone H3K4 trimethylation helps to recruit the TFIID complex. (2) Mediator bridges interactions between activators and the basal initiation machinery, and can stimulate basal transcription as well. At some promoters Mediator can recruit TFIIH and TFIIE independently of RNApoII. (3) TBP can also be brought to promoters by the SAGA complex. In S. cerevisiae, this pathway is most utilized at TATA containing promoters. The Mot1 and NC2 complexes can repress this pathway by actively removing TBP from the TATA element. (4) Mot1 and NC2 can also have a positive role in transcription by removing non-productive TBP complexes from DNA, thereby allowing functional PICs to form. Regulatory sequences expand in number and complexity with increased complexity of the organism ~ 30-100 bp ~ 100s bp Could be 50kB or more Are distant enhancers in proximity to the promoter? Chromosome conformation capture ( 3C) 3C is a variant of ChIP. Cells are treated with formaldehyde to create DNA-protein-DNA cross-links. (Formaldehyde reacts with the amino groups on proteins and nucleic acids to form protein-protein and DNA protein covalent linkages). The DNA is then treated with a restriction nuclease that produces cohesive ends. Prior to the ligation step, the DNA is diluted so that the DNA ligase will join two different DNA fragments only if they are cross-linked. Finally, the cross-links are reversed and the DNA is purified, so that the ligated DNA molecules can be quantified by PCR. 3C reveals proximity of enhancers and promoters eg. colocalization in the ligated DNA product; eliminating transcription of a gene eliminates colocalization of enhancer and promoter sequences ( eg. b-globin locus) Why are enhancer and promoter in close proximity during active transcription? = Nucleosomes Looping via protein-protein Interactions; intervening DNA loops out; suggestive that the direct interactions mediate activation RNA polymerase “transcription factories”; in higher eukaryotes, some evidence that transcription occurs at a limited number of positions; co-localization could be result of activation Enhancers can promote chromatin modification over large distances Groudine Cell 144: 327 ( 2011) The RNA Polymerase II CTD (or tail) Heptad repeat unit YSPTSPS P P P proline can be cis or trans Cis-proline is well suited to making hairpin turns in polypeptide chains. COOH NH2 5 repeats in plasmodium 26 repeats in yeast 52 repeats in mammals Regions upstream (R1) and downstream (R3) of the heptad repeat region are enriched in the submotifs What is the role of the Pol II CTD? Mouse RNA Pol II wt 52 5 50 hrs. HeLa cells Introduce CTD construct CTD - amaR examine RNAs Splicing, processing of 3’ end, termination were all affected - amanitin Nature 385: 357 (1997) How the Polymerase CTD Couples Transcription to other processes Kinase/ phosphatase TF II H, mediator YSPTSPS P Factors recruited capping factors elongation pTEFb (Cdk9) In S. cerevisiae, shared by Cdk1 and Bur 1 YSPTSPS P P Further elongation phosphatases (Rtr1(2?) YSPTSPS Phosphatases (Fcp1, ssu72) splicing components histone methylase DNA repair enzymes P 3’ end processing factors Termination YSPTSPS Mediator, activators, GTFs TECs are community organizers The major steps in mRNA processing (trx, 5’ capping, polyA addition, splicing) all occur together on a transcript extruded from the exit channel of RNAP although they can be reconstituted independently in vivo Principles of “cotranscriptionality” to integrate nuclear metabolism 1.Permits coupling between different biogenesis steps; eg crosstalk; suspected when decreasing one step has effects on 2nd; could always be indirect a. Landing pad—increase concentration of reactants—proteins involved in capping etc b. Allosteary: guanosyl transferase of capping enzyme activated by interaction with phosphorylated CTD c. Kinetic coupling—optimize timing 2. Impose order or control a. Juxtaposition of proteins permits assembly, competitive interactions handoffs; often mutually exclusive PPis b. directions emanating from phopshorylation state of CTD 3. A locator for nuclear machines –DNA repair, modification etc Bentley: Cotranscriptionality Mol cell rev 2009 Fast elongation favors exon skipping whereas slow elongation favors exon inclusion De la mata What don’t we know about the CTD? 1. What is the role of Ser-7 phosphorylation? Ser-7 shows high phosphorylation across highly transcribed protein coding genes in S. cerevisae, but no role yet ascribed to this modification 2. What is the significance of different markings when comparing noncoding and protein coding genes and how is this difference set up? 3. To what extent do interdependent and co-occurrence of marks set-up bivalent/multivalent recognition patterns 4. Genome wide ChIP analysis indicates some factors thought to be recruited by Ser-2 phosphorylation appear either signficantly prior to or after that event. Explanation? See Tietjen…..Ansari NMSB(2010) 17: 1154 Mayer….Cramer NMSB (2010): 1272 What is known about the role of Spt4/5 in elongation control? Spt5: essential in yeast A promoter proximal pause is characteristic of transcription of many genes in higher eukaryotes Characteristics of paused polymerase ( pioneering work by John Lis Hsp70 locus in Drosophila) 1.In open complex ( KMnO4 footprinting) 2.Some fraction can elongate (nuclear run-on experiments) Later work: 3. Ser-5 phosphorylated on CTD 4. Spt4/5 (DSIF) and NELF associated with paused polymerase ( ChIP; + required to recapitulate pause in vitro ) Paused polymerase What triggers release to productive elongation? 1)pTEFb phosphorylates polymerase CTD, Spt5, NELF 2) Backtracking relieved ( SII) 3) NELF dissociation Genome wide studies sequencing 5’ capped short mRNAs found them associated with ~30% of all genes in Drosophila; positions of their 3’ ends correspond to positions of stalled polymerase, and were also regions of high GC content; length of short mRNAs increases when SII is depleted suggesting that paused polymerases had backtracked and their mRNAs had been cleaved by SII Adelman, Science, Cell 2010 Potential roles of Paused Polymerase NRG ( 2012) 13: 720 Spt4/5 is also connected to other elongation complexes Using activity based assay, Spt4/5, PAF and Tat-SF1 required for efficient elongation (DNA template) Spt4/5 PAF Phosphorylation of Spt5 CTD by Bur-1 required for PAF entry into elongation complex Tat-SF1 Physical interaction PAF Using chromatin template and completely reconstituted factors, PAF stimulates elongation synergistically with TFIIS (independent of other activities of the PAF complex) PAF TFIIS ∆PAF ∆TFIIS Physical interaction Synthetic lethal Each elongation factor also interacts with RNAP Coupling between Transcription and Translation through Rpb 4/7 Stalk Stalk 1.Rpb4/7: eukaryotes and archae 2.Loosely associated with RNAP 3.Not essential 4.In molar excess over RNAP subunits Bacterial RNAP Eukaryotic RNAP Evidence 1.Physical/genetic interaction between Rpb4/ EIF3 2. Rpb4 physically associated with polysomes 3. ∆ Rpb4/7 cells have abnormal polysomes and are sensitive to translation inhibitors 4. Rpb4 association with polysomes contingent on its association with RNA polymerase