What elements from the periodic table support all life on Earth?

UNIT 1 –
UNDERSTANDING
LIFE ON EARTH
What qualifies something as living?
• Organisms are made up of cells
• Organisms reproduce sexually or asexually and some can do both
• Organisms will have a genetic code, DNA
• Organisms will grow and develop
• Organisms will obtain and use energy
• Organisms will respond to their environment
• Organisms will strive to maintain homeostasis
• Organisms will change over time
Discussion
• What kinds of cells?
• What is an example of asexual reproduction?
• What is an example of sexual reproduction?
• If all organisms have DNA is that DNA identical?
• What is the difference between grow and develop?
• What are 2 ways organisms obtain energy?
• What does homeostasis mean?
• What if living things do not adapt to changes in their environment?
Answers:
• Prokaryote-bacteria Eukaryote-plant , animals
• Mitosis is asexual reproduction
• Meiosis is sexual reproduction
• No the DNA is not identical, except in identical offspring.
There are similarities but variation in the species
• Growth is to add more cells, Develop is to mature into and adult
• Organisms can obtain energy by being either an autotroph or heterotroph
• Homeostasis means same-state. Organisms need to maintain a stable internal environment in the body
• If an organisms does not adapt to changes in the environment then they must change the environment or die
Evolution
• Can be sudden or gradual
• Sudden changes that interrupt the gradual progress is called
Punctuated Equilibrium.
• Explanation:
• https://www.youtube.com/watch?v=6QiRoO8af7s
Levels Life is organized
• Atoms
• Molecules
• Cells *life begins here
• Tissue
• Organ
• Organ System
• Organism
• Species
• Population
• Community
• Ecosystem
• Biosphere
INTRO TO BIOCHEMISTRY
What elements from the periodic table support all life on Earth?
• CARBON
• HYDROGEN
• OXYGEN
• NITROGEN
• PHOSPHORUS
8
Periodic Table of Elements
• Highlight the following element on your Periodic Table.
The table should be glued in on the Left.
• Carbon
• Hydrogen
• Oxygen
• Nitrogen
• Phosphate
• Sulfur
Carbon-based Molecules:
Organic chemistry: study of carbon compounds
Carbon has 4
electrons in an outer energy level
that holds eight;
Can form 4 covalent bonds with many other
10
Shape of Organic Molecules:
shape=functi on
determines its function in an organism
11
Giant Molecules – Polymers:
• Large molecules are called polymers
• Monomers link together to form larger molecules called polymers
• Biologists call polymers macromolecules or biomolecules
12
Linking Monomers:
Cells link monomers by removing a molecule of water this process is called dehydration synthesis
.
Remove H
H
2
O Forms
Remove OH
13
Breaking Down Polymers:
• Cells break down macromolecules by adding a molecule of water
• this process is called hydrolysis
14
Macromolecules in
Organisms:
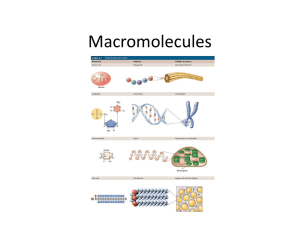
There are four macromolecules:
Carbohydrates
(CHO)
Lipids (CHO)
Proteins (CHON)
Nucleic Acids (CHONP)
15
CARBOHYDRATES
16
Monosaccharides:
• Monosaccharides are the monomers of carbohydrates; also called simple sugars
• Examples: glucose, fructose, & galactose
• Chemical Formula for monosaccharides is C
6
H
12
O
6
; this a ratio of 1:2:1
• Monosaccharides are the main fuel that cells use for cellular work; they are a source of quick energy
• Disaccharides (2 Sugars bonded);
Polysaccharides:
Large sugar molecules; take longer for body to break down
• Starch is an example of a polysaccharide in plant cells
• Glycogen is a polysaccharide found in animal cells
• Starch and glycogen are extra amounts of sugar taken in by the cell and stored for later use
• Cellulose is a polysaccharide found in
plant cell walls; most abundant
18
LIPIDS
Lipids:
• Lipids are hydrophobic –”water
fearing”; they do not mix with water
• Includes fats, waxes, steroids and oils
• Functions –
store energy
Insulate body
Cushion and protect organs
Form cell membranes
20
Structure of Lipids:
• Triglyceride - Monomer of lipids
Composed of 1 glycerol molecule and
3 fatty acid chains
Glycerol forms the “backbone” of the triglyceride
• Triglycerides are composed mainly of
carbon and hydrogen; oxygen is found only in the glycerol molecule
21
Lipids in Organisms:
• Most animal lipids exist as solids at room temperature (butter, lard, fat
layer on steak/chicken, waxes)
• Most plant lipids tend to exist as liquids at room temperature
(peanut, sunflower, canola oils)
22
Lipids & Cell Membranes:
• Cell membranes are made of phospholipids
• Phospholipids have a
head that is polar; it attracts water
(hydrophilic)
• Phospholipids also have 2
tails that are nonpolar and do not attract water
(hydrophobic) 23
Steroids:
• Cholesterol is the
“base steroid” from which your body produces other steroids
• Estrogen and
testosterone are examples of these other steroids
24
PROTEINS
Proteins:
• Proteins are large, folded polymers made of monomers called amino acids
• Elements in proteins: C, H, O and N
• Functions:
Build cells
Act as hormones
Act as enzymes
Cellular transport
26
Linking Amino Acids:
• This process is done by the ribosomes in the cell by removing a water
molecule from the amino acids
• The process is called a condensation or
dehydration reaction; forms peptide bonds
27
Enzymes are proteins
• What do enzymes do?
• Enzymes control the rate of chemical reactions
• Enzymes are also referred to as biological catalysts
• enzymes work by weakening bonds and lowering the amount of activation
energy needed for the reaction
• Enzymes act on a substrate; they are specific to substrate
28
Enzyme + Substrate =
Product:
29
NUCLEIC ACIDS
Nucleic Acids
• Store hereditary information
• Contain information for making all the body’s proteins
• Elements in nucleic acids: C, H, O,
N and P
• Types of nucleic acids: DNA and
RNA
31
• Nucleic acids are polymers;
Nucleotides are the monomers
• Nucleotides are composed of:
5-carbon sugar phosphate group nitrogeneous base
Nitrogeneous Bases:
• Each DNA nucleotide has one of the following bases:
– Adenine (A)
– Guanine (G)
– Thymine (T)
– Cytosine (C)
• Each RNA nucleotide has one of the following bases:
– Adenine (A)
– Guanine (G)
– Uracil (u)
– Cytosine (C)
33
Shape of dna and rna:
• One strand of RNA forms a single helix
• Two strands of
DNA join together to form a double helix
34
ATP
• ATP is the energy currency of cells
• Made of a nucleotide with 3 phosphate groups
35