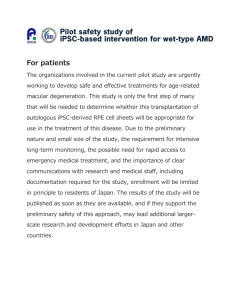
Age-related macular degeneration and the Y402H polymorphism in
advertisement

Manchester Biomedical Research Centre Age-related macular degeneration and the Y402H polymorphism in complement factor H Tony Day Simon Clark Progression of AMD Age-related maculopathy Soft drusen + pigmentary changes in RPE Age-related macular degeneration Atrophic Neovascular AMD is a condition that primarily affects RPE cells in the macula Why particularly the macula? • Most metabolically active • Light damage Ageing changes within the RPE • • • • Intracellular accumulation of lipofuscin Autofluorescent material that accumulates in lysosomes Contains lipid and protein Formed from incompletely degraded photoreceptor outer segments and autophagy • Induces free-radical formation Accumulation of extracellular material derived from RPE Drusen Diffuse thickening of Bruch’s membrane 2 micron Retinal pigment epithelium Druse RPE cells Bruch’s membrane Choroid Thickening of Bruch’s Membrane (basal linear deposits) Extracellular material disrupts transport between RPE and choroid and thereby increases RPE stress Ageing retina and progression to AMD High metabolic activity, light, oxygen resulting in free radical formation and RPE dysfunction Genetic susceptibility Inflammation and accumulation of intracellular and extracellular debris Environmental factors Critical point reached Death of retinal/RPE cells Atrophy Choroidal neovascularisation What predisposes to AMD apart from ageing? • Genetic risk factors: Major risk factors • Complement factor H (especially Y402H polymorphism) • ARMS2/HTRA1 locus (Chr 10q26) Intermediate risk factors • Complement factor 2/complement factor B locus • Complement factor 3 Minor risk factors • ABCA4 • ApoE Decreased risk CFHR1/CFHR3 deletion • Environmental influences • • • • Smoking ↑ Abdominal adiposity ↑ Anti-oxidant intake ↓ Omega-3 fatty acid intake ↓ Complement cascade * * * * Cellular damage, phagocytosis, inflammation Implicated in AMD Functions of Complement Factor H (CFH) There is tick over C3b is deposition on host surfaces which can amplify and lead to activation of alternative pathway unless suppressed CFH is a soluble protein mainly produced by the liver that binds to polyanionic structures (such as glycosaminoglycans (GAGs)) on host surfaces and prevents C3b mediated complement activation Structure of CFH CFH consists of 20 complement control protein (CCP) modules Y402H polymorphism is a major genetic risk factor for AMD Facilitate decay of C3 convertase Cofactor for factor I 1 4 CRP C3b GAG/polyanion 6 7 8 GAG/polyanion Cell surface C3b, C3d 12 14 19 20 402Y/H 402 residue contributes to GAG binding site (Prosser et al., J. Exp Med 2007) Glycosaminoglycans (GAGs) Heparan sulfate (HS) proteoglycans Heparin a highly sulfated form of HS made specifically by mast cells Types of GAGs Immunolocalisation of CFH in human macula tissue (OX23/24 Abs) RPE Drusen Bruch’s membrane Hypothesis and research question • Hypothesis – differential binding of 402H and 402Y forms to GAGs explains the association of the polymorphism with AMD (still debate as to whether this is the functional change associated with this genetic locus) • Question – Do the 402H and 402Y forms show differential binding to (normal) macular tissue Methodology 6 H 8 402H CCP6-8 (disease associated) 6 Y 8 402Y CCP6-8 H Full length CFH 402H Y Full length CFH 402Y • 402H and 402Y CCP6-8 (recombinant) or CFH (full-length) were fluorescently-labelled (H - red) (Y - green) and applied in pairs to frozen sections of “normal” post mortem human macula tissue (age 46-90) • Relative levels of binding of the 402H and 402Y forms were calculated after measuring fluorescence (and subtracting background fluorescence) • In some experiments tissue sections treated with GAG degrading enzymes prior to application of fluorescently-labelled proteins Results 402H and 402Y CCP6-8 and full-length CFH merge 402Y RPE CCP6-8 100 µm Bruch’s membrane Relative fluorescence (arbitrary units) 402H 200 CCP6-8 6 7 8 402H 6 7 8 402Y * 150 100 50 402H 402Y 402H 402Y 0 Bruch’s membrane RPE Note similar pattern in choroidal vessels 402Y merge flCFH Relative fluorescence (arbitrary units) 402H H CFH 402H Y CFH 402Y flCFH 100 ** 80 60 40 20 402H RPE Control experiments: Binding unaffected by genotype of tissue Results unaffected by exchanging fluorophores Unlabelled proteins effectively competed for binding 402Y 402H 402Y 0 Bruch’s membrane * P = 0.0005, ** P = 0.009 402H and 402Y (CCP6-8) binding sites are predominantly heparan sulphate (HS) with a smaller amounts of dermatan sulphate (DS) Relative binding of 402H and 402Y from previous slide No evidence of interactions with hyaluronan or chondroitin sulfate Effects of heparinise and chondroitinase B digestion on binding of full length 402H and 402Y and CFH antibody labelling Binding of CCP6-8 Y and H variants to selectively desulfated heparin (Heparin is a highly sulfated form of HS) Conclusion 1. 402H form of CFH binds poorly to Bruch’s membrane and choroidal structures compared to 402Y form 2. CFH binds particularly to HS and the differing affinities of 402H and 402Y forms and dependent upon HS sulfation patterns 3. Suggests a possible disease mechanism….. 402Y form localises to Bruch’s membrane and thereby protects against complement activation on the membrane 402H form localises poorly to Bruch’s membrane and this may lead to complement over-activation, inflammation and eventually AMD 4. Supports the Y402H amino acid substitution being the genetic alteration that predisposes to AMD.