Protein Synthesis: Translation of
the Genetic Message
Chapter Twelve
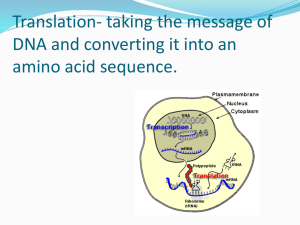
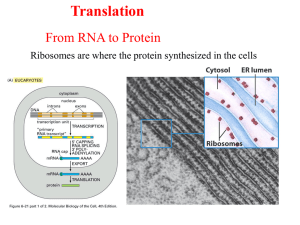
Translating the Genetic Message
• Protein biosynthesis is a
complex process requiring
ribosomes, mRNA, tRNA, and
protein factors
• Several steps are involved
• Before being incorporated into
growing protein chain, a.a.
must be activated by tRNA and
aminoacyl-tRNA synthetases
Salient features of the genetic code
– Triplet: a sequence of three bases (a codon) is needed
to specify one amino acid
– Nonoverlapping: no bases are shared between
consecutive codons
– Commaless: no intervening bases between codons
– Degenerate: more than one triplet can code for the
same amino acid; Leu, Ser, and Arg, for example, are
each coded for by six triplets
– Universal: the same in viruses, prokaryotes, and
eukaryotes
The Genetic Code
• The ribosome moves
along the mRNA
three bases at a time
rather than one or
two at a time
The Genetic Code
• All 64 codons have
assigned meanings
– 61 code for amino acids
– 3 (UAA, UAG, and UGA)
serve as termination
signals
– only Trp and Met have
one codon each
The Genetic Code
• The third base is irrelevant
for Leu, Val, Ser, Pro, Thr,
Ala, Gly, and Arg
• Amino acids coded by 2, 3,
or 4 triplets - the third
letter of the codon - Gly, for
example, is coded by GGA,
GGG, GGC, and GGU
Wobble Base Pairing
• Some tRNAs bond to
one codon exclusively,
but many tRNAs can
recognize more than
one codon because of
variations in allowed
patterns of hydrogen
bonding
– the variation is called
“wobble”
– wobble is in the first
base of the anticodon
Base Pairing Combination in the
Wobble Scheme
If there are 64 codons, how can there
be less than 64 tRNA molecules?
• The wobble hypothesis provides insight
– in many cases, the degenerate codons for a given
amino acid differ only in the third base; therefore
fewer different tRNAs are needed because a given
tRNA can base-pair with several codons
– the existence of wobble minimizes the damage that
can be caused by a misreading of the code; for
example, if the Leu codon CUU were misread as CUC
or CUA or CUG during transcription of mRNA, the
codon would still be translated as Leu during protein
synthesis
Amino Acid Activation
• Amino acid activation
and formation of the
aminoacyl-tRNA take
place in two separate
steps
• Both catalyzed by
aminoacyl-tRNA
synthetase
• Free energy of
hydrolysis of ATP
provides energy for
bond formation
tRNA Tertiary Structure
• There are several recognition sites for various
amino acids on the tRNA
Chain Initiation
• In all organisms, synthesis of polypeptide chain
starts at the N-terminal end, and grows from Nterminus to C-terminus
• Initiation requires:
–
–
–
–
–
–
tRNAfmet
initiation codon (AUG) of mRNA
30S ribosomal subunit
50S ribosomal subunit
initiation factors IF-1, IF-2, and IF-3
GTP, Mg2+
• Forms the initiation complex
The Initiation Complex
Chain Initiation
– tRNAmet and tRNAfmet contain the triplet 3’-UAC-5’
– Triplet base pairs with 5’-AUG-3’ in mRNA
– 3’-UAC-5’ triplet on tRNAfmet recognizes the AUG
triplet (the start signal) when it occurs at the
beginning of the mRNA sequence that directs
polypeptide synthesis
– 3’-UAC-5’ triplet on tRNAmet recognizes the AUG
triplet when it is found in an internal position in
the mRNA sequence
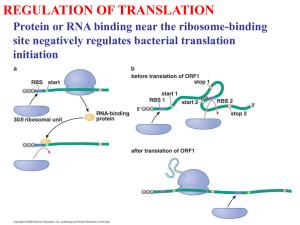
How does the ribosome know where
to start translating
– Start signal is preceded by a Shine-Dalgarno
purine-rich leader segment, 5’-GGAGGU-3’
– Lies about 10 nucleotides upstream of the AUG
start signal and acts as a ribosomal binding site
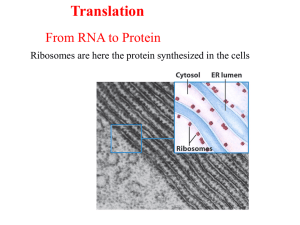
Chain Elongation
• Uses three binding sites for tRNA present on the 50S
subunit of the 70S ribosome: P (peptidyl) site, A
(aminoacyl) site, E (exit) site.
• Requires
–
–
–
–
70S ribosome
codons of mRNA
aminoacyl-tRNAs
elongation factors EF-Tu (Elongation factor temperatureunstable), EF-Ts (Elongation factor temperature-stable),
and EF-G (Elongation factor-GTP)
– GTP, and Mg2+
Chain Elongation
Why is EF-Tu so important in E.coli?
• Involved in translation fidelity
• tRNA and aminoacid are mismatched then EFTu will not bind to t-RNA and will not deliver it
to ribosome
• Binds activated tRNA too well and does not
release it from ribosome
Elongation Steps
• Step 1
– an aminoacyl-tRNA is bound to the A site
– the P site is already occupied
– 2nd amino acid bound to 70S initiation complex. Defined by the mRNA
•
Step 2
– EF-Tu is released in a reaction requiring EF-Ts
• Step 3
– the peptide bond is formed, the P site is uncharged
• Step 4
–
–
–
–
the uncharged tRNA is released
the peptidyl-tRNA is translocated to the P site
EF-G and GTP are required
the next aminoacyl-tRNA occupies the empty A site
Chain Termination
• Chain termination requires
– stop codons (UAA, UAG, or UGA) of mRNA
– RF-1 (Release factor-1) which binds to UAA and UAG
or RF-2 (Release factor-2) which binds to UAA and
UGA
– RF-3 which does not bind to any termination codon,
but facilitates the binding of RF-1 and RF-2
– GTP which is bound to RF-3
• The entire complex dissociates setting free the
completed polypeptide, the release factors, tRNA,
mRNA, and the 30S and 50S ribosomal subunits
Chain Termination
Components of Protein Synthesis
Protein Synthesis
– In prokaryotes, translation begins very soon after
mRNA transcription
– It is possible to have several molecules of RNA
polymerase bound to a single DNA gene, each in a
different stage of transcription
– It is also possible to have several ribosomes bound to
a single mRNA, each in a different stage of translation
– Polysome: mRNA bound to several ribosomes
– Coupled translation: the process in which a
prokaryotic gene is being simultaneously transcribed
and translated
Simultaneous Protein Synthesis on
Polysomes
• A single mRNA molecule is translated by
several ribosomes simultaneously
• Each ribosome produces a copy of the
polypeptide chain specified by the mRNA
• When protein has been completed, the
ribosome dissociates into subunits that are
used again in protein synthesis
Simultaneous Protein Synthesis on
Polysomes
Eukaryotic Translation
• Chain Initiation:
• the most different from process in prokaryotes
• 13 more initiation factors are given the designation eIF
(eukaryotic initiation factor) (Table 12.4)
Eukaryotic Translation
• Chain elongation
– uses the same mechanism of peptidyl transferase and
ribosome translocation as prokaryotes
– there is no E site on eukaryotic ribosomes, only A and
P sites
– there are two elongation factors, eEF-1 and eEF-2
– eEF2 is the counterpart to EF-G, which causes
translocation
• Chain termination
– stop codons are the same: UAG, UAA, and UGA
– only one release factor that binds to all three stop
codons
Posttranslational Modification
• Newly synthesized polypeptides are frequently modified
before they reach their final form where they exhibit
biological activity
– N-formylmethionine in prokaryotes is cleaved
– leader sequences are removed by specific proteases of the
endoplasmic reticulum; the Golgi apparatus then directs the
finished protein to its final destination
– factors such as heme groups may be attached
– disulfide bonds may be formed
– amino acids may be modified, as for example, conversion of
proline to hydroxyproline
– other covalent modifications; e.g., addition of carbohydrates
Examples of Posttranslational
Modification
Protein Degradation
• Degradative pathways are restricted to
– subcellular organelles such as lysosomes
– macromolecular structures called proteosomes
Protein Degradation
• In eukaryotes,
ubiquitinylation
(becoming bonded to
ubiquitin) targets a
protein for destruction
– those with an N-terminus
of Met, Ser, Ala, Thr, Val,
Gly, and Cys are resistant
– those with an N-terminus
of Arg, Lys, His, Phe, Tyr,
Trp, Leu, Asn, Gln, Asp, Glu
have short half-lives
Acidic N-termini Induced Protein
Degradation
•
•
•
This project is funded by a grant awarded under the President’s Community Based Job Training Grant as implemented by the
U.S. Department of Labor’s Employment and Training Administration (CB-15-162-06-60). NCC is an equal opportunity
employer and does not discriminate on the following basis:
against any individual in the United States, on the basis of race, color, religion, sex, national origin, age disability,
political affiliation or belief; and
against any beneficiary of programs financially assisted under Title I of the Workforce Investment Act of 1998
(WIA), on the basis of the beneficiary’s citizenship/status as a lawfully admitted immigrant authorized to work in the United
States, or his or her participation in any WIA Title I-financially assisted program or activity.
Disclaimer
• This workforce solution was funded by a grant awarded under the
President’s Community-Based Job Training Grants as implemented by the
U.S. Department of Labor’s Employment and Training Administration. The
solution was created by the grantee and does not necessarily reflect the
official position of the U.S. Department of Labor. The Department of Labor
makes no guarantees, warranties, or assurances of any kind, express or
implied, with respect to such information, including any information on
linked sites and including, but not limited to, accuracy of the information
or its completeness, timeliness, usefulness, adequacy, continued
availability, or ownership. This solution is copyrighted by the institution
that created it. Internal use by an organization and/or personal use by an
individual for non-commercial purposes is permissible. All other uses
require the prior authorization of the copyright owner.