ecture 3: the building blocks of life
advertisement

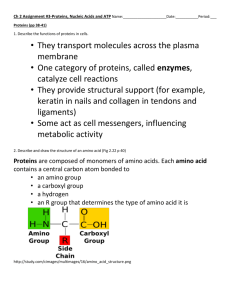
Building Blocks of life Molecular Structure: DNA, RNA and amino acids Lecture 3 Chromosome to DNA molecule • A chromosome is “essentially” a long strand of dsDNA (double stranded Deoxyribonucleic acid) wound around proteins; e.g. histones, and condensed to form a structure called chromatin. • However it order for the DNA to carry out its function is must be unwound from the proteins: i.e. chromatin -> long strand of dsDNA • This dsDNA strands are shaped in the form of a “double Helix” • Each DNA strand consists of nucleotides which are joined together. • The DNA “double Helix” is two such strands which are coiled and connected together via what are referred to as: nucleotide bases or bases The Crick and Watson double Helix • The dsDNA has the following important features: • I strand, The primary/sense strand, runs from 5’ to 3’ • The corresponding strand, the complimentary/antisense strand, runs 3’ to 5’ • The bases (nucleic acids) joining the strands; • Base pairings (always the same pairings): – A (adenine) <-> T (Thymine) – C (Cytosine) <-> G (Guanine) Adapted from [1] p.194 [you can find the original article by C and W p 195] RNA: Ribose nucleic acid • A molecule closely associate with DNA and which a part of the “gene expression” process is referred to as RNA • The RNA [nucleotide] is very similar to DNA [nucleotide] except: – Its nucleic acid has a ribose sugar as opposed to a deoxyribose sugar. – The Nucleotide base Thymine is replace with an equivalent base called Uracil (klug p.191) – The RNA strand is single stranded Amino acids • The third important molecule, associated with genetics, is the “amino acid” • An amino acid is a molecule that has two main elements: a constant part [shown in pink in the next slide] and a variable part. This variable part has specific chemical properties which are essential to its function. • In proteins [chains of amino acids] the constant regions are referred to as the “backbone” (main chain) and the variable region as the side chains. AMINO Acids (AA) and their properties • Amino acids are grouped according to properties, refer to diagram. • These properties help determine the final shape of proteins • For example hydrophobic amino acids [non polar] tend to stay away from water and are in the centre of proteins • Hydrophilic [polar] tend to be on the outside surface of proteins • Two other important amino acids to note are: cystine which has a sulphur (S) in the side chain • Tryptophan which is the largest amino acid. An amino acid chain (Polypeptide) • When two AA are joined together “peptide” bonds • A number of AA joined by peptide bonds are called a polypeptide chain. • The polypeptide has two elements: the main chain connected via peptide bonds; and side chains (associated with functionality or determine the final shape). Secondary and Tertiary structure • The primary chain (polypeptide chain) then begins to change its shape depending on the side chain properties of the amino acid to firstly form: • the secondary structure: consisting of α helixes and β sheets both • The secondary structure then changes to the tertiary structure • The diagram shows a myoglobin [like haemoglobin] molecule with α helixes and β sheets, the heme group [blue] contains iron and does not contain AA and red ball an oxygen molecule Secondary to Tertiary structure • Secondary structures interact with the environment to form the tertiary or 3-D structure of the protein : essentially the polypeptide chain is contorted to form the most thermodynamically stable structure: • Since most proteins are in water A number of factors affect the formation: 1. Polar (Hydrophilic) AA try to stay on the outside of the structure 2. Non polar (hydrophobic) stay on the inside • However in some cases the proteins are in hydrophobic solution [Lipids in the cell membrane] and in this case the structure would alter: non polar outside, polar inside. Exam question • Describe, using suitable examples, the three important molecules associated with the existence of life. [this will form part of a question] References • [1] Klug 7th ed • [2] Http://biotech.matcmadison.edu/resources/proteins /labManual/chapter_2.htm; accessed on the 21/9/2011