1.5 proteins and nucleic acids notes 2012
advertisement

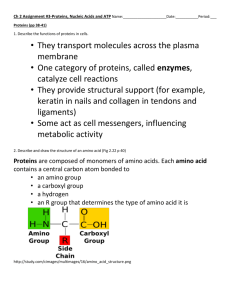
1.5 PROTEINS ( polypeptides) Nelson page 39–44, do que 1– 7 pg 47 - very important biological molecules making up about 50% of the dry cell mass - amino acid polymers in specific 3-D shape - USES: structural, control metabolism (enzymes), membrane transport, hormones, recognition and receptors on membranes AMINO ACIDS: monomers made of carboxyl and amino groups around a central carbon with a variable side group (R). - 20 amino acid with 20 different “R” - groups - 8 essential A.A. are not made by humans so must be in the diet PEPTIDE BONDS: covalent bonds formed by dehydration between amino acid (-NH2) of one amino acid and the carboxyl (-COOH) of a second amino acid. Polypeptide 50 or more amino acids in length (can be 1000’s long). A Protein is one or more polypeptides that are folded into a precise 3dimensional shape called its conformation which can be filamentous (linear), sheet, or globular. Genes on DNA directs ribosomes to join Amino Acids together in a specific sequence in a process called Translation making specific proteins. GLOBULAR PROTEINS (make your own notes) - 4 levels of structure primary, secondary, tertiary, quaternary - denaturing can destroy the function of proteins 1.5 NUCLEIC ACIDS Nelson page 45 – 47 do questions 8 – 11 page 47 informational macromolecules that store hereditary information that determine structural and functional characteristics Includes two types: Deoxyribonucleic Acid DNA (double stranded helix) and ribonucleic acid RNA (single stranded helix) both are polymers made of subunits called nucleotides Nucleotides General Structure of a Nucleotide Nucleotides have five possible organic bases attached as functional units : Adenine (A) and Guanine(G) are double ring Purines Thymine (T), Cytosine (C) and Uracil (U) are single ring Pyrimidines DNA only contain A, G, C, T while RNA has A, G, C, U DNA has a carbon backbone of the sugar deoxyribose (lacks Oxygen on carbon #2) RNA has a carbon backbone of ribose Nucleotides are connected together to form a polymer called a strand The Nucleotides are covalently bonded (phosphodiester bond) together between the phosphate of one group to the hydroxyl of the next group The two strands in DNA are held together by H-Bonds between specific organic bases on the adjacent strand which runs the opposite direction (antiparallel) Adenine forms 2 H-bonds with only Thymine Guanine forms 3 H-bonds with only Cytosine Other types of Nucleotides: Adenosine triphosphate ATP Contains three phosphates and used to drive almost all energy requiring reactions in the cell have three highly energetic phosphate bonds for short term energy storage Nucleotide derivatives include: o Nicotinamide adenine dinucleotide (NAD+) and o Flavin adenine dinucleotide (FAD) used as electron acceptors and short term energy transporters during cellular respiration o NADP+ is used in photosynthesis o cyclic adenosine monophosphate (cAMP) is used as a second messenger during hormone interactions and gene control