Period 1 Presentation
advertisement

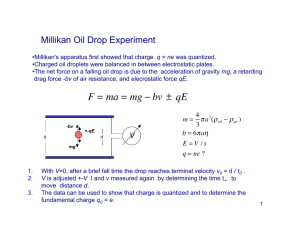
Robert Millikan Born March 22,1868 in Morrison, Ill. Died on December 19,1953in San Marino, California. Attended Oberlin College in Ohio. (1886-1891) Obtained master degree in physics at Colombia University in 1893. Worked as a physics teacher. Professor at the University of Chicago for 11 years. He discovered accurate charge of an electron using “Falling drop method.” Verified Einstein's photoelectric equation. Won the Nobel Prize for physics in 1923. Experimental Design The oil is sprayed into the chamber above the two parallel charged plates. The oil drops through a hole in the top plate. X rays cause the air particles to lose electrons which then stick to the oil droplets. This causes the oil droplets to have a negative charge. Millikan could control the rate of the droplets charge by varying the intensity of the electric field. (Basically, he could make the oil droplets float in mid-air using invisible X rays.) Postulates of Theory Millikan figured out that the magnitude of each drop's charge increased slightly and he determined that the smallest common denominator was 1.602 x10^-19 coulombs. (The coulomb is the unit for electric charge.) In order to make things easier, Millikan made 6.02 x10^-19 equal to 1-. From there, he based everything else off of that. From knowing Thomson's charge-to-mass ratio, Millikan then figured out the mass of an electron. (Look at page 109 in the textbook for equation.) Works Cited Buthelezi, Thandi, et al. Chemistry: Matter and Change. New City: McGraw Hill Glencoe, 2008. York The Nobel Foundation. "Robert A. Millikan Robert Millikan: The Nobel Prize in Physics 1923."Nobel Prizes. The Nobel Foundation. 22 Oct. 2007 <http://nobleprize.org/nobelprizes/physics/laureates/1923/millikanbio.html>. "Determination of the Charge on the Electron: Thomson and Robert Millikan." Atomic Structure Table of Contents. ChemTeam. 23 Oct. 2007 <http://dbhs.wvusd.k12.ca.us