Millikan`s Oil Drop Experiment
advertisement

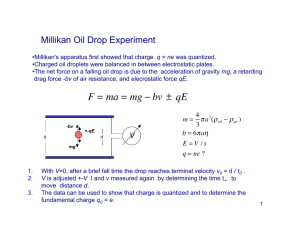
Millikan’s Oil Drop Experiment PHYSICS CHAPTER 22 C Atoms By the early 1900s, we knew: Atoms had electrons in them (J. J. Thomson) Atoms had positive charges in them (protons) Structure??? Millikan’s Experiment Milikan Oil Drop Millikan Millikan’s Experiment Experiment balanced gravity and charges Eq = mg If E is known due to voltage (V = Ed) And weight is determined We can find q Q was found to be a multiple of 1.6 x 10-19 C Example p375 An oil drop weighs 1.9 x 10-14 N. It is suspended in an electric field of intensity 4.0 x 104 N/C. What is the charge on the oil drop? What we know: W = 1.9 x 10-14 N E = 4.0 x 104 N/C Equation: Q = W/E Substitute: Q = (1.9 x 10-14 N)/(4.0 x 104 N/C) Math: Q= 4.75 x 10-19 C Example p 375 continued If the drop is attracted toward the positive plate, how many excess electrons does it have? What we know: Q = 4.75 x 10-19 C e- charge: 1.6 x 10-19 C Equation: # e- = q/charge of e- Substitute: #e- = (4.75 x 10-19 C)/(1.6 x 10-19 C) Math: #e- = 2.9688 = 3