The Elementary Charge
advertisement

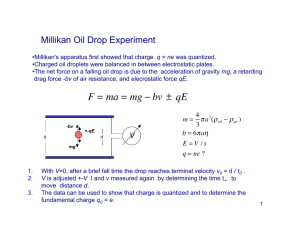
Day 3.7 Measuring and Controlling Electrons 1) Millikan performed an experiment that determined that all charges are made of whole number multiples of an elementary – or smallest – electrical charge. To model how he did this you will do a similar experiment. There are many identical containers holding random numbers of identical ball bearings. a) Find the mass of one ball bearing without opening the containers. Describe your steps. b) There are only a small number of containers. Why would it be better to have more? c) The scale is only accurate to 1 g. How can you calculate a mass that is accurate to 0.1 g? 2) Watch Millikan`s Oil Drop Experiment http://www.youtube.com/watch?v=XMfYHag7Liw a) Draw a diagram of the apparatus. b) Why did Millikan use oil drops when they are so hard to see? c) What were the x-rays for? d) What did he learn by observing them fall without any charge? e) Why did he adjust the voltage? f) Draw force diagrams of the oil drop falling at terminal velocity and when it is still. 3) Watch the Millikan Oil Drop Sonata http://www.youtube.com/watch?v=SxcxN_UVuFI . a) Why do you let the oil settle? b) When the screen says ‘down’, all go down. When it says ‘up’ some still go down. Why? c) Find an example of a drop that is held stationary by the field. When does it occur? 4) Go to Exploring the Millikan Oil Drop Experiment http://webphysics.davidson.edu/applets/pqp_preview/contents/pqp_errata/cd_errata_fixes/section4_5.html a) b) c) d) e) Why do the drops fall up? Why was a microscope used? Get an oil drop to be stationary Describe what you had to do to succeed. Record the voltage. Measure the terminal velocity of the drop. The lines are 0.1 mm apart. Terminal velocity is given by v = 2 r2 g/9, where is the viscosity of air (7.25 x 10-6 Ns/m2) and is the density of the oil (875 kg/m3). Calculate the radius and mass of the drop. f) The charged plates are 6 mm apart. Calculate the charge on the drop. How many elementary charges does it have? 5) Do Lab Exercise 7.5.1 page 374: Part 2 Textbook: 7.5 p. 362 #4 - 6, p. 364 # 3 – 5