Charge of an Electron Worksheet Key
Name:____________ KEY __________________Date:_____________________Period:_____
Charge of an Electron
Worksheet Key
Background
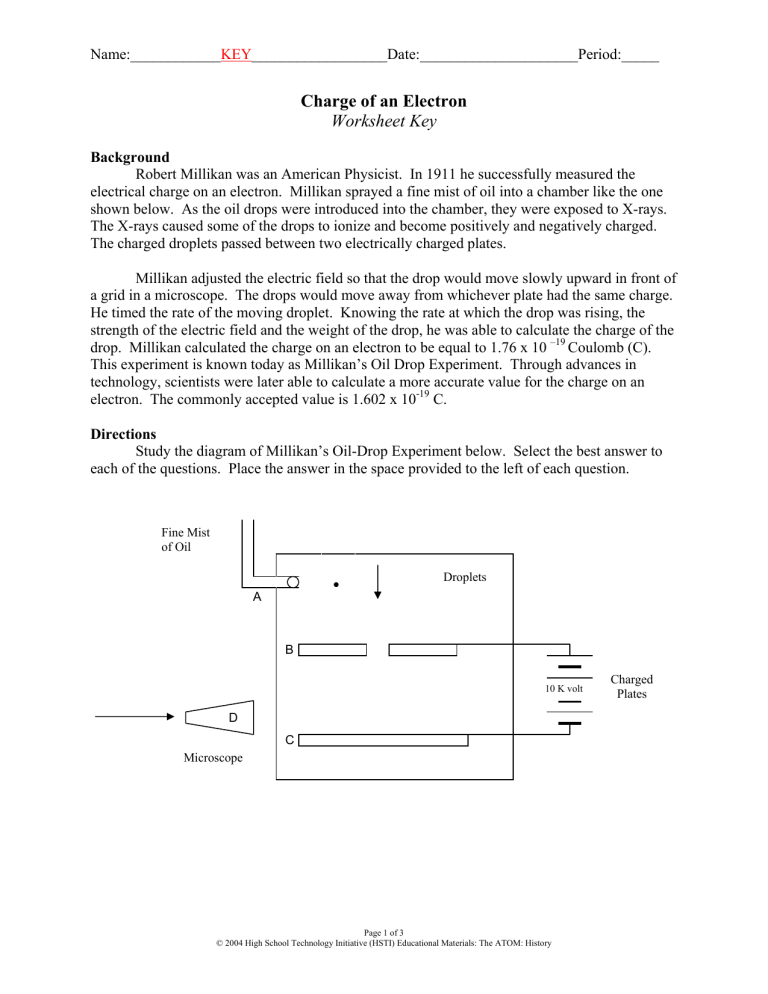
Robert Millikan was an American Physicist. In 1911 he successfully measured the electrical charge on an electron. Millikan sprayed a fine mist of oil into a chamber like the one shown below. As the oil drops were introduced into the chamber, they were exposed to X-rays.
The X-rays caused some of the drops to ionize and become positively and negatively charged.
The charged droplets passed between two electrically charged plates.
Millikan adjusted the electric field so that the drop would move slowly upward in front of a grid in a microscope. The drops would move away from whichever plate had the same charge.
He timed the rate of the moving droplet. Knowing the rate at which the drop was rising, the strength of the electric field and the weight of the drop, he was able to calculate the charge of the drop. Millikan calculated the charge on an electron to be equal to 1.76 x 10
–19
Coulomb (C).
This experiment is known today as Millikan’s Oil Drop Experiment. Through advances in technology, scientists were later able to calculate a more accurate value for the charge on an electron. The commonly accepted value is 1.602 x 10
-19
C.
Directions
Study the diagram of Millikan’s Oil-Drop Experiment below. Select the best answer to each of the questions. Place the answer in the space provided to the left of each question.
Fine Mist of Oil
A
.
Droplets
B
D
Microscope
C
10 K volt
Charged
Plates
Page 1 of 3
© 2004 High School Technology Initiative (HSTI) Educational Materials: The ATOM: History
Name:____________ KEY __________________Date:_____________________Period:_____
B 1. What is the purpose of device ‘A’?
(a) To create an electrical potential difference (voltage) between the plates.
(b) To introduce a fine mist of liquid oil into the chamber.
(c) To magnify the droplets of the oil.
(d) To locate positively charged particles.
C 2. What is the purpose of device ‘D’?
(a) To create an electrical potential difference (voltage) between the plates.
(b) To introduce a fine mist of liquid oil into the chamber.
(c) To magnify the droplets of oil.
(d) To locate positively charge particles.
A 3. To cause a positively charged oil droplet to move upward, what charges would plates ‘B’ and ‘C’ possess?
(a) B= a negative charge; C= a positive charge
(b) B= a positive charge; C= a negative charge
(c) Both ‘B’ and ‘C’ have a neutral charge
(d) B= a deficiency of electrons; C= an excess of electrons
D 4. What situation would cause a droplet to be momentarily suspended between the two electrical plates?
(a) The droplet had a neutral charge.
(b) The plates were not charged.
(c) The mass of the electrons in the droplet equaled the mass of the protons.
(d) The repulsive and attractive electrical forces of the plates exactly balanced the mass of the droplet.
D 5. What information did Millikan use to determine the charge on an electron?
(a) The rate at which the oil drop was rising from the plate.
(b) The strength of the electric field.
(c) The mass of the oil drop.
(d) All of the above.
Page 2 of 3
© 2004 High School Technology Initiative (HSTI) Educational Materials: The ATOM: History
Name:____________ KEY __________________Date:_____________________Period:_____
Optional
By using the charge to mass ratio (e/m) of an electron, Millikan was able to calculate the approximate mass of an electron. Show below how Millikan calculated the approximate mass of an electron. Use the current value given for the charge of an electron. e/m = 1.76 x 10
8
C/g
1g (
-19
)
-28
1.602 x 10 C = 9.10 x 10 g
8
1.76 x 10 C
Page 3 of 3
© 2004 High School Technology Initiative (HSTI) Educational Materials: The ATOM: History