Particles & Quantum Physics (AQA Unit 1)
advertisement

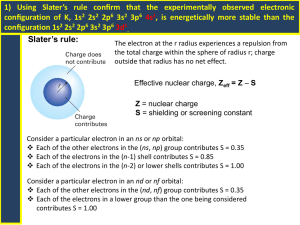
AQA Physics A Unit 1: Particles, Quantum Phenomena and Electricity Dr K. A. Newson Head of Physics TGSG 2008 Overview of New AS Physics Course Unit 1 Unit 2 Unit 3 (ISA) 75 minute exam. 75 minute exam. Internally assessed practical (½) % of total AS level marks 40 40 20 % of total A level marks 20 20 10 How is it examined? Physics A Grading System Unit 1 Unit 2 Unit 3 (ISA) AS Qualification Marks Grade Grade Marks Grade Total Marks 96 -120 A A 48 - 60 A 240 - 300 A 84 - 95 B B 42 - 47 B 210 - 239 B 72 - 83 C C 36 - 41 C 180 - 209 C 60 - 71 D D 30 - 35 D 150 - 179 D 48 - 59 E E 24 - 29 E 120 - 149 E 0 - 47 U U 0 - 23 U 0 - 119 U Grade New A* Grade New A* grade is for complete A level, it is not awarded for the AS course alone. To gain an A* grade students must both: Gain at least 270 (90%) Uniform marks on the A2 Gain at least 480 (80%) Uniform marks overall on the A level course. Overview of Unit 1 1 Matter and Radiation 2 Quarks and Leptons 3 Quantum Phenomena 4 Electric Current 5 Direct Current Circuits 6 Alternating Currents Matter and Radiation 1.1 Inside the atom 1.1 Inside the atom Aims of lesson Understand the structure of the atom and nucleus, Develop your knowledge of the constituent particles that make up the atom/nucleus Know what is meant by an Isotope and how to represent them Prior knowledge Every atom consists of a small positively charged nucleus surrounded by negatively charged electrons; The nucleus consists of neutrons and protons which are roughly equal in mass; Protons and electrons have equal but opposite charges, neutrons are uncharged. Large and small Physics deals with the smallest sub-nuclear particle to the entirety of the Universe. Physicists need to use a vast range of values, to make this easier prefixes are used: For example, a high energy particle collider may accelerate protons to energies of 3000000000eV, its far simpler and less prone to error to simply say 3GeV. In this case G means Giga which means 109 eV. The full list of prefixes is given to you as a handout. 1.1 Inside the atom The basic particles... Proton positive charge Neutron no charge Electron negative charge Describing the atom Atomic Number - the number of protons 3 Neutron Number - the number of neutrons 4 Mass (nucleon) Number - the number of nucleons + = 7 Properties of protons, neutrons and electrons Property → Particle ↓ Mass (relative to p) Charge (relative to p) Proton p 1.67 x10-27 kg 1 amu (u) + 1.6 x 10-19C Nucleon (part of nucleus) (+1e) Neutron N 1.67 x10-27 kg 1 amu (u) Electron e 9.1 x10-31 kg 1/1800 amu Description 0 Nucleon - 1.6 x 10-19C (-1e) Found in a cloud around the nucleus Isotopes Isotopes are atoms of the same element i.e. that have the same number of protons, but have different numbers of neutron. Examples of Isotopes For example, there are 3 isotopes of hydrogen: 1 1) H (hydrogen) 1 2) 2 1 H (AKA deuterium) 3 H (AKA tritium) 1 In the case of Tin (Sn) there are 13 isotopes from 3) 112 50 Sn to 125 50 Sn Nuclear structure Key words and terminology Atomic number (Z): the number of protons contained in the nucleus (AKA proton number). Mass/Nucleon number (A): the number of protons and neutrons contained in the nucleus. Neutron number (N): the number of neutrons contained in the nucleus. N=A-Z Isotope Notation A quick way of representing isotopes is to use the following system: Mass number (A) Atomic number (Z) U 92 235 Element’ s chemical symbol Specific Charge The word ‘Specific’ in Physics means ‘per unit mass’, The specific charge therefore is given by: Specific charge (Ckg-1) = Charge (C) Mass (kg) E.g. the specific charge on a proton is found by: specific charge = 1.60 x 10-19 ÷ 1.67 x 10-27 = 9.58 x 107 Ckg-1 Example a) b) Find the specific charge of the following: Electron of mass 9.11 x 10-31 kg An Aluminium ion (Al3+) of mass 4.51 x 10-26 kg Answers a) 1.76 x 1011 Ckg-1 b) 1.06 x 107 Ckg-1 Nuclear Stability Aims of lesson Understand that some isotopes are unstable and examine the causes of this. Know about the three types of radioactive decay and what happens to the nucleus in each case Prior knowledge Radioactive substances emit radiation because they are unstable. Know about the 3 types of ionising radiation and their properties e.g. their penetrating abilities. Nuclear Stability Protons and neutrons make up the nucleus. However, the protons are all positively charged; so how can they form a nucleus when they should repel one another? The reason why nuclei do not fall apart arises from a force which will be new to you….. This new type of force is called the STRONG NUCLEAR FORCE (for obvious reasons), it is also called the Strong interaction. It is one of the four Fundamental forces. Fundamental Interactions (forces) There are four fundamental interactions, and all forces in nature can be attributed to these four: 1.Gravitational 2.Electromagnetic 3.Weak nuclear 4.Strong nuclear Fundamental forces Force Relative Range strength (fm) Additional Notes 1 10-1 Electromagnetic 10-3 ∞ Weak nuclear 10-16 10-3 Gravitational 10-41 ∞ Strong nuclear Force between nucleons and quarks. Force between charged particles Force controlling beta decay (see later notes) Force between masses Forces in the nucleus The strong nuclear force holds protons and neutrons together in the nucleus. The strong nuclear force has a very short range typically about 4 fm (1 fm = 10-15m). At distances larger than this it is insignificant and the repulsive electromagnetic force dominates. At very short distances ~0.5 fm the strong nuclear force becomes repulsive to stop protons and neutrons being pushed into each other. The heaviest unstable nuclides can be alpha emitters “Strong” force holds the nucleons together “Electromagnetic (coulomb) force” forces protons apart Strong force must be greater than electromagnetic force for stability Larger stable nuclides N>Z Excess of neutrons…therefore Beta minus emitters Excess of protons…therefore Beta plus emitters Smaller stable nuclides N=Z Three types of ionising radiation all come from the nucleus Beta Electrons (or positrons) Gamma Electromagnetic wave Alpha 2 protons, 2 neutrons Useful websites http://durpdg.dur.ac.uk/lbl/particleadventure/in dex.html Electromagnetic waves and Photons Electromagnetic Waves Electromagnetic spectrum The Electromagnetic spectrum The Electromagnetic (EM) spectrum is the entire range of EM waves (radiations). Properties of EM radiation All types consist of vibrating magnetic and electric waves (handout), the two waves are in phase, that is they vibrate together. All EM waves are transverse, that is the vibration is at right angles to the direction of the wave’s motion. All travel with the same speed (3.00 x 108 ms-1) in a vacuum. Radio waves Microwaves Infra-red Visible light Ultra-violet X-rays Gamma-rays The EM Spectrum Frequency Hz Wavelength m Radio < 400 x 106 ~ 1 - 1000 Microwave ~ 2.5 x 109 ~ 0.1 – 0.01 Transmitters Rotating molecules ~10-24 J ~ 1013 - 1014 ~ 10-6 Hot objects, LEDs ~ 1 eV (1.6 x 10-19 J) Type Infra-red Origin Oscillating currents in aerials ~ (7 – 4) x 10-7 Very hot objects, LEDs Photon energy ~10-28 – 10-25 J ~2 – 3 eV Visible ~ 5 x 1014 UV > 7.5 x 1014 < 4 x 10-7 Extremely hot objects, discharge tubes, > 3 eV X-rays ~ 1018 ~ 10-10 Stopping high energy electrons. ~ 104 eV Gamma rays ~ 1020 ~ 10-12 Nuclear decay ~106 eV Cosmic rays >>1020 Very short From distant parts of galaxy. ~10 J Photons EM waves are emitted in packets called Photons and energy Max Planck (1901) concluded that the energy carried by light and the other types of electromagnetic radiation existed in discrete packets called Quanta. The energy E carried by each quantum is given by: E = hf Where f is the frequency (in Hz), h is a constant called the Planck constant. The value of Planck’s constant is 6.63×10-34 Js. Photons and energy In 1905 Einstein proposed that light radiation consists of a stream of quanta called Photons. Example: Q) What is the energy of a photon of red light that has a wavelength of 650nm? Answer: As frequency (f) = speed of light (c) ÷ wavelength (λ) f = c/λ, therefore the energy E = hf = hc/λ Therefore E = (6.63×10-34 × 3×108) ÷ (6.5×10-7) E = 3.1×10-19 J Q) What is the energy of violet light (λ = 400nm) Answer E = 5×10-19 J Example 1. 2. What are the photon energies of the following EM radiations: X-ray photon of frequency of 2.5 x 1019 Hz A photon of blue light having a wavelength of 475nm. Answers 1. E = hf = (6.63 x 10-34 x 2.5 x 1019) = E = 1.66 x 10-14 J 2. E = hf = hc/λ = (6.63 x 10-34 x 3.00 x 108/4.75 x 10-7) E = 4.19 x 10-19 J Laser power A laser beam consists of a stream of monochromatic (same f and λ) photons. The power of a laser = energy transferred each second by the photons. If the energy of a single photon = hf Then the laser power = nhf Where n = number of photons emitted per second The electron volt The electron volt (eV) is defined as the energy gained by an electron when it is moved through a potential difference of 1Volt. 1eV = 1.6 x 10-19J Sometimes the mass of particles is given in terms of their mass-energy in units of eV. This arises because of the relationship between mass and energy (E = mc2). For example, the mass of an electron (me) is given by: Me = 9.1 x 10-31kg = 8.19 x 10-14 J = 0.511 MeV More about this later. Rest energy Einstein said that the mass of a particle when stationary, its rest mass (m0), has an energy called its rest energy which is locked up as mass. The rest energy is given by E = m0c2 Particles and antiparticles Consider the following decay: 11 6C 11 0 + 5 B + +1 β This is called beta+ decay, the beta+ particle is usually known as a positron. It is virtually identical to an electron other than its charge which is positive. The positron is the antimatter equivalent of an electron. We call it the antiparticle of the electron. Particles and antiparticles Carl Anderson 1932 Tracks of positrons in a cloud chamber, identical to normal beta (β-) particles but bent in the opposite direction indicating the charge on a positron is opposite to beta particles. Particles and antiparticles The existence of antimatter was predicted by the English Physicist Paul Dirac in 1928 According to Dirac’s theory for every particle there exists a corresponding antiparticle. Such antiparticle has exactly the same rest mass but the opposite charge (if the particle is charged). The particle and antiparticle pair annihilate each other if they meet converting their rest-mass into photons of gamma radiation. Annihilation of positron and electron pair An electron and positron meet and they release two gamma rays. E = mc2 When a particle and its anti-particle meet their mass is converted to radiation energy by the equation E = mc2. Two photons are produced, this is needed for conservation of momentum. We can calculate the frequency of the gamma rays by equating the photon energy to the mass energy of the positron-electron pair. See next slide. E = mc2 and annihilation The mass-energy of particles is sometimes expressed in units of eV The energy of each gamma photon = hf If each electron has a mass of 0.511 MeV Then 0.511MeV = 9.1 x 10-31Kg so equating the energies: mec2 = hf therefore f is given: f = mec2 /h = 1.23 x 1020Hz (so λ = (c/f) = 2.42 x 10-12m Pair production Pair production is basically the reverse of annihilation. In pair production a photon creates an electron and a positron and disappears in the process. Pair production Incident photon (gamma ray) Pair production The electron and positron follow curved trajectories in a magnetic field. How can we tell from the tracks that they have opposite charges? Energy and Pair production The energy of each gamma photon = hf If each electron or positron has a mass of 0.511 MeV Then the energy of the energy of the gamma photon must be equal to 2 x 0.511 MeV. I.e. the photon must have an energy of 1.022MeV, this is equal to 1.08 x 10-13J f = E /h = 2.46 x 1020Hz So λ = (c/f) = 1.21 x 10-12m Particle interactions Interaction of particles An interaction is the process by which two particles exert forces on each other. In other words momentum is transferred between the particles (if no other forces act). For example, as two electrons approach one another they repel each other and move away from each other. The Physicist Richard Feynman found that the force between the two particles is caused by the exchange of an exchange particle in this case a virtual photon. The term virtual means they cannot be detected directly and if we did detect them this would stop the force from acting. Feynman Diagrams Feynman represented the interaction of particles my means of a Feynman diagram. For example, the electromagnetic interaction between two electrons. The paths do not show the paths of the particles. The virtual photon (γ) is represented by a wave. _ _ Future e e γ Time _ e Past Exchange particle is a virtual photon γ _ e Feynman diagrams _ e Future γ Time _ e Past _ e Exchange particle is a virtual photon γ _ e Analogy See analogy with skaters on page 13 of text book Weak nuclear force (weak interaction) The electromagnetic force results from the exchange of a virtual photon between the two charged particles. What about beta decay? What force is at work? The force responsible for beta decay is the weak nuclear force (interaction). Beta +/- decay At GCSE you given a simplified version of what goes on in beta decay. In reality there is an addition particle emitted with the beta +/- particle. This particle is called the neutrino (ν) _ (and antineutrino ν). 11 6C 11 0 + 5 B + +1 β + 14 6C _ 11 0 7 N + -1 β + ν ν Beta plus decay Beta minus decay Neutrinos Neutrinos are very low mass neutral particles which do not interact very much other particles. Neutrinos and antineutrinos interact by means of the weak interaction (see later notes). Weak interaction and W bosons The exchange particle involved with the weak interaction is called the W boson. W bosons have: Non-zero rest mass (unlike photons) Very short range (~ 0.001fm) Are charged, W+ is positively charged whilst W- are negatively charged. Feynman diagrams for B- decay Note that in the Feynman diagrams charge is conserved. In β− decay, the weak interaction converts a neutron (n) −) and an into a proton (p) while emitting an electron (β _ antineutrino (ν). Recall that a proton consists of uud quarks, a neutron consists of udd quarks. So β− decay is due to the conversion of a down quark (d) to an up quark (u) by emission of a W− boson; the W− boson subsequently decays into an electron and an antineutrino. Feynman diagrams for B- decay _ ν Feynman diagrams for B+ decay In β+ decay, the weak interaction converts a proton (p) into a neutron (n) while emitting a positron (β+) and an neutrino (ν). Recall that a neutron consists of udd quarks, a proton consists of uud quarks. So β+ decay is due to the conversion of an up quark (u) to a down quark (d) by emission of a W+ boson; the W+ boson subsequently decays into an positron and an neutrino. Feynman diagrams for B- decay t n _ ν udd W+ udu P Neutrino interactions Neutrinos rarely interact with other particles: However a neutrino can interact with a neutron making it turn into a proton by means of the weak interaction. The process also releases a β− particle. In equation form: 1 0 n+ ν W - 1 1 p+ β 0 -1 Feynman diagrams for neutronneutrino interaction p Future WTime n Past e _ _ (b) Exchange particle is a W- boson n Antineutrino interactions Antineutrinos interact in a similar way: An antineutrino can interact with a proton making it turn into a neutron by means of the weak interaction. The process also releases a β+ particle. In equation form: 1 1 _ p+ ν W + 1 0 n+ β 0 +1 Feynman diagrams for protonantineutrino interaction n Future + e W+ Past (b ) Exchange particle is a W+ boson Time p + _ n Another weak interaction Electron capture A proton in a proton rich nucleus may turn into a neutron by interacting through the weak interaction with an inner shell electron form outside the nucleus. The W+ boson turns the electron into a neutrino. 1 p + 1 0 -1 e- W + 1 n + ν 0 Feynman diagram for electron capture n Future n W+ Time p Past e _ Summary of Weak interactions The exchange particle is the W+ or W- boson W+ or W- bosons have a non-zero mass W+ or W- bosons are charged When a proton interacts with the weak interaction the W+ boson is the exchange particle. When a neutron interacts with the weak interaction the W- boson is the exchange particle. Charge is always conserved in all weak interactions Building blocks of nature Fundamental particles are particles which are believed to have no substructure, that is particles which cannot be split into smaller particles. Hadrons are particles composed of fundamental particles known as quarks. For each type of quark there exists an antiquark The quark family There are thought to be 6 types (or flavours) of quarks, arranged in 3 groups called generations: Quark type 1st generation 2nd generation 3rd generation = Up (u)* Charge (p =1) +⅔ Down (d)* -⅓ = Strange (s)* +⅔ Charm (c) -⅓ = Top (t) Bottom (b) * Only need to worry about these quarks. +⅔ -⅓ More about quarks For each type of quark there exists an anti-quark. The charge on an anti-quark is the opposite to its quark equivalent. For example a up quark (u) has a charge of +2/3, _ therefore an anti-up quark ( u ) has a charge of -2/3. Protons are _made up of 3 quarks (2u and 1d). Therefore antiprotons p are made up of 2anti-up and 1 anti-down ___ quark ( uud ). Particle families We can categories all particles in the following manner: All particles Matter and antimatter Hadrons Leptons Baryons Mesons (3 quarks) (quark and antiquark) Hadrons Particles (and antiparticles) that interact by the strong interaction (and electromagnetic interaction if charged) are called hadrons. They decay through the weak interaction with the exception of protons which are stable. They are composed of quarks (and antiquarks). Hadrons may be further divided into two categories: Baryons and Mesons. Baryons Baryons all consist of a combination of 3 quarks (antibaryons consist of 3 antiquarks). The quarks are bound together by the strong interaction. It is impossible to isolate a solitary quark. Baryons include the familiar protons and neutrons, but also include more exotic particles…see table in hand out. Mesons All known mesons are believed to consist of a quark- antiquark pair. There are approximately 140 types of quark. For example a pion p- consists of a down quark and an anti-up quark (du). See handout for table of examples. Leptons Leptons are a family of particles that do not interact through the strong interaction, they include electrons and neutrinos, see full list in hand out. Leptons do interact through the weak interaction and, if charged, through the electromagnetic interaction. There are 3 types of lepton (and the antiparticle), each type has its own associated neutrino Leptons are thought to be fundamental, that is they cannot be broken down into simpler particles. Leptons τ Name Symbol Charge (p) Electron/(positron) e- / e + -1 / +1 Electron ν ν 0 e e neutrino/antineutrino Muon/(antimuon) μ- / μ+ -1 / +1 Muon ν ν 0 μ μ neutrino/antineutrino - / τ+ -1 / +1 τ Tauon/(antitauon)* Tau* neutrino/antineutrino ντ ν-τ 0 Mass (MeV) 0.511 >0 105.5 >0 1777 >0 Particle Collisions Many of the exotic particles are studied by colliding beams of particles and antiparticles into each other. It is important that the conservation of mass-energy is applied: The total mass-energy of particles and antiparticles does not change after the collision. That is: Total energy = Rest energy of particles + Kinetic energy of particles Particle Collisions Therefore by applying the conservation of energy (mass): Rest energy of = Total energy products before collision _ Kinetic energy of products Particle collisions Knowing the rest energy of the products is this gives information as to the possible identity of the products. For example: if we have a collision between a proton and an antiproton each with a rest energy of 1GeV and if each has a kinetic energy of 2GeV, the total energy is given by: Total energy before collision (Etot) Etot = Total rest energy + Total kinetic energy = (2 + 2) + (1 + 1) = 6 GeV Therefore the resulting collision could produce a range of products as long as the conservation rules are applied and the products total energy does not exceed 6 GeV. Lepton number conservation Leptons are assigned a lepton number = +1 Antileptons are assigned a lepton number = -1 Non-leptons are assigned a lepton number = 0 In any interaction the total lepton number is conserved. Example of lepton number conservation 1 Consider the two interactions: νe + n Lepton number = Lepton number = → p + +1 0 0 νe and + n _ +1 0 → X p 0 e-1 + Allowed Because lepton number is conserved e+ Not Allowed -1 Because lepton number is not conserved Example of lepton number conservation 2 Muon decay: which of the following decays is allowed? Use the lepton number conservation rule to find out. μ- → e- + or μ- → e- + _ νe + νμ _ _ νe + νμ Example of lepton number conservation Consider the two interactions: μ- → eLepton number = +1 +1 νe + νμ +1 -1 +1 and _ _ e μ- → X Lepton number = + _ +1 + νe -1 + Because lepton number is conserved νμ -1 Allowed Not Allowed Because lepton number is not conserved Lepton branches Consider the muon decay below: μ → ? e- + νe + _ νμ The above change satisfies both the rules regarding the conservation of charge and lepton number. But this change is never seen. Why? Remember there 3 branches (families) of lepton, each branch has the particle together with its neutrino and their antiparticles. The conservation rule must be applied to each lepton branch in any interaction. Therefore, a muon must produce muon neutrino and not an antineutrino. So you must check that each family has its lepton number conserved. K mesons (Kaons) Strange particles were discovered in experiments in which protons (or neutrons) and pions (π mesons) collide with each other. They were called strange particles because they decayed into through the weak interaction to give either: 1. π mesons, or 2. π mesons and protons 1. Particles that decayed to give only π mesons were called K mesons. 2. Particles that decayed to give π mesons and protons were sigma (Σ) particles. See diagram K mesons (Kaons) π meson K meson π meson (green) collides with proton (red) π meson π meson Σ (sigma) particle proton Quarks and Antiquarks 1 π- + p → K+ + Σ- Was observed 2 π+ + n → K+ + Σ0 Was observed 3 π- + n → K0 + Σ- Was observed 4 π- + n → K- + Σ0 Was not observed Conservation of strangeness!! Why were some reactions observed but not others? Remember that one of the six flavours of quark is the strange quark. To explain why some reactions are not observed it is necessary to introduce another conservation rule: the conservation of strangeness number (S) Strange quarks have a strangeness number of -1, strange antiquarks have a strangeness number of +1, all other quarks have a strangeness number of zero. In any strong interaction strangeness is always conserved. Strangeness in the quark family Quarks Up Charge (p=1) Strangeness S Antiquarks Down Strange Up Down Strange _ d _ s u d s _ u +⅔ -⅓ -⅓ -⅔ +⅓ +⅓ 0 0 -1 0 0 +1 Conservation of strangeness!! 1 π- + p → K+ + Σ- Was observed 2 π+ + n → K+ + Σ0 Was observed 3 π- + n → K0 + Σ- Was observed 4 π- + n → K- + Σ0 Was not observed Explanation of interactions Using the conservation of strangeness it is easy to see why some reactions were observed and others were not. For example, in reaction 1 below: π- + p → K+ + Σ The π- and p both have S = 0, the K+ has S = +1 and the Σ- has S = -1, so strangeness is conserved. However, consider reaction 4 below: π- + n → K- + Σ0 The π- and p both have S = 0, the K- has S = -1 and the Σ0 has S = -1, so strangeness is conserved. Conservation of baryon number A further conservation rule covers baryons. Baryon numbers are assigned as follows: +1 for baryons -1 for antibaryons 0 for leptons and mesons. (another way to consider this is that quarks have a baryon number of 1/3 and antiquarks have a baryon number = -1/3) In any reaction the total baryon number is always conserved Example of baryon number conservation Consider the reaction: This reaction is observed because it obeys the conservation of baryon number rule. I.e. p = 1, p = -1, and π+ and π- both = 0, so both sides = 0. However, consider the reaction: _ p + p → π+ + π- _ p + p → p + π+ This reaction is not observed …..Why? In terms of baryon numbers, the left side has a total of 0, but the right side has a baryon number totalling 0. What other conservation rule is disobeyed? Answer: Charge Chapter 3: Quantum phenomena Atomic Spectra Electrons in an atom can only have only certain specific energies. These energies are called the electron energy levels. An energy level diagram shows the possible energies for an electron in a given atom. For example: the energy level diagram for hydrogen 0 eV Ionisation level -0.8 eV Second excited state -3.4 eV First excited state Energy (eV) -1.5 eV -13.6 eV Ground state Energy changes Absorption of energy causes an electron to go a higher energy level. The atom gains energy. Emission of energy causes the electron to fall to a lower energy level. The atom loses energy. Ground state and excitation of electrons Normally electrons occupy the lowest energy levels available. The atom and electrons are said to be the ground state. The electron can absorb energy; this causes it to move to a higher energy level. The atom is said to be in an excited state. A precise amount of energy is needed to excite an electron from one level to another. The energy is equal to the difference in energy between the two levels. Example if an electron in a hydrogen atom is excited from the ground state to the first excited state it must absorb energy (ΔE) equal to E2 – E1 ΔE -3.4 eV First excited state -13.6 eV Ground state = -3.4 - -13.6 = 10.2 eV Emission contd. An electron in an excited state must lose energy for it to move back down to the ground state. The energy must again be exactly equal to the difference in energy between the two levels. Example: in the above electron energy level for hydrogen, how much energy (ΔE) must an electron in the 2nd excited state lose for it to fall directly back to the ground state? ΔE = energy of the excited state – energy of the ground state ΔE = -1.5 – (-13.6) = 12.1 eV Emission Under usual conditions, an excited electron will only temporarily remain excited. Excited electrons soon return to the ground state, this may be directly (A) or via another energy level (B). A B -1.5 eV 2nd Excited state (E3) -3.4 eV 1st Excited state (E2) -13.6 eV Ground state (E1) Ionisation energy Energy (eV) 0 eV Ground state (-13.6 eV for H) e.g. for hydrogen the ionisation energy = 13.6eV Points regarding ionisation Electrons in the highest energy level are defined as having zero energy. Consequently, all other energy levels are negative. Free electrons have only kinetic energy and their total energy is always positive. Allowable changes and electron transition The energy levels for an atom are fixed and are unique for that particular chemical element. All atoms of the same element have the same energy levels. Electron excitation Electrons can be excited to higher energy levels by many different ways: Usually by the absorption of electromagnetic radiation Also by: collision with other particles (e- impact) heating Important point The energy (ΔE) needed to excite an electron to a higher energy level is exactly the difference in energy between the two levels, i.e. ΔE = E2 – E1 E2 Excited state E1 Initial state Important point If the excitation is caused by the absorption of a photon, the photon energy (hf) must be equal to the difference in energy between the two energy levels, i.e. hf = ΔE = E2 – E1 If the excitation is caused by electron impact, the electrons kinetic energy does not have to match ΔE as any surplus kinetic energy is retained by the colliding electron. i.e. KEinitial = ΔE + KEfinal Emission of energy When an electron falls to a lower energy level it rids itself of the surplus energy (ΔE) by emitting a photon of EM radiation. The value of ΔE is defined as being the exact difference between the energies of the two energy levels (E1 and E2). Therefore: ΔE = (E1 - E2) = hf Ionisation If an electron can gain enough energy to reach the highest energy level, it can leave the atom (is free). The atom is said to be ionised. The ionisation energy is defined as: the energy required to completely remove an electron (in its ground state) from an atom. Emission Spectra Because the atoms of each element have a characteristic set of energy levels, they will emit a set of unique characteristic frequencies. Analysis of the frequency (or λ) of the light emitted can be used to identify the element. The group of frequencies of radiation emitted are called a line spectrum. Each element has its own unique spectrum. The atomic hydrogen spectrum Types of spectra Quantum Physics Part 2 The Photoelectric effect The Photoelectric effect Free electrons are held in a metal by the electrostatic attraction of the positively charged nuclei. If an electron is to be removed from the surface of a metal it must be given energy. Photoelectric emission is the release of electrons from the surface of a solid (metal) when electromagnetic radiation is incident on the surface. Photoemission Incident EM radiation Released electrons (photoelectrons) Metal The work function (Φ) The minimum amount of energy to remove an electron from a substance is called the work function (symbol Φ). Different materials have different work functions: Material Caesium Potassium Lithium Zinc Work function (J) 3.43 × 10-19 3.6 × 10-19 3.71 × 10-19 6.6 × 10-19 Threshold frequency To remove the electron from the material, the photon energy (hf) must be at least equal to the work function. The lowest frequency at which the photon energy is sufficient to remove an electron is called the threshold frequency (fmin). i.e. hfmin = Φ Threshold frequency If the frequency of the EM radiation is less than the work function no electron is released. The condition for photoelectron emission is: hf ≥ Φ Note: in calculations sometimes a maximum wavelength that corresponds to the threshold frequency is given, this is called the cut-off wavelength (λo). λo = c ÷ fmin Einstein’s Photoelectric equation For photoelectric emission, the photon energy must be at least equal to the work function of the material. What happens to the excess energy if the photon energy is greater than the work function? The answer is that the excess energy is taken by the released electron as kinetic energy (Ek). Conservation of energy gives Einstein’s equation: Einstein’s Photoelectric equation Max kinetic energy of electron photon Work = function energy So Ek = hf – Φ Example 3.0 eV Photon arrives Electron emitted with kinetic energy of 0.7 eV Lithium metal Φ = 2.3 eV Example 2 Light of frequency 6.7 × 1014 Hz shines onto clean Caesium metal. What is the maximum kinetic energy and speed of the electrons emitted? The work function of caesium metal is 3.43 × 10-19J and Planck’s constant is 6.63× 10-34 Js. Electron emitted with kinetic energy = ? Photon frequency = 6.7 × 1014 Hz Caesium Φ = 3.43 × 10-19 J Answer Using Einstein’s equation gives Ek = hf – Φ Ek = (6.63× 10-34 × 6.7 × 1014) – 3.43 × 10-19 Ek = 1.01 × 10-19 J As Ek = ½mv2, this gives v2 = 2Ek/m therefore v = 4.71× 105 ms-1 Photoelectric effect experiments A simple photocell may be used to determine a value for the Planck constant (h). Apparatus: Light of known frequency f _ pA R ( E Photoelectric cell V + Method The potentiometer R is set so that the voltmeter reads zero. Light is directed onto the emitting electrode (E). If frequency f ≥ fo photoelectrons are emitted. The release of the photoelectrons is detected by the current flowing through the picoammeter. The voltage across the photocell is increased until the current decreases to zero. This voltage value is called the stopping potential (Vs). The stopping potential is recorded for a range of frequencies. The photocell The work done in moving a charge q through a potential difference V is given by: Work done = qV The energy needed to reach the collector electrode (c) = qV _ + c Determination of Planck’s constant The energy needed to reach the collector electrode = qV Where q = electron charge (1.6 ×10-19C) Where V is the voltage across the photocell V is increased until the electrons cannot reach the collector. The stopping potential Vs is the voltage when electrons fail to reach the collector. At this stage: KE of electron = qVs (1) Determination of Planck’s constant From Einstein’s photoelectric equation: Electron KE = ½mv2 = hf – Φ From equation (1) : qVs = hf – Φ Therefore: Vs Φ hf = – q q and Vs = hc qλ – Φ q Determination of Planck’s constant Using the equations: Vs hf = q – Φ q or Vs = hc qλ – Φ Q Allows the value of Planck’s constant to be determined. This is achieved by recording the stopping potential and either the corresponding frequency or wavelength. Determination of Planck’s constant Stopping potential (Vs) If the stopping potential Vs (Y-axis) is plotted against the frequency f (or 1/λ) (X-axis), the graph can be used to determine Planck’s constant h. Gradient of graph = h/q fo Φ/q Photon frequency (Hz) Note, if the X-axis is 1/λ, the gradient is hc/q Saturation and photocurrents The photoelectrons are released from the surface with a range of kinetic energies. If the collector electrode has a negative potential not all the electrons will reach the collector (their kinetic energy < qV). However, if the collector electrode potential is made positive, the photocurrent increases (as more electrons are collected). At the saturation potential ALL the electrons are collected. Saturation and photocurrents + c Photocurrent - Vs _ Saturation 0 + Voltage Frequency, current and intensity The intensity (brightness) of the light determines the number of photons that are incident on the surface. The greater the intensity, the higher the photocurrent (providing that f ≥ fo). Photocurrent Saturation bright dim Vs 0 Voltage V Frequency, current and intensity (II) If red and blue lights of equal intensity are used, the resulting graph would be: Photo-current Red light Blue light Vs Voltage V The blue light has a higher (-)Vs as it has a higher photon energy. Frequency, current and intensity (II) The intensity of the two radiations is the same. This means that both the red and blue lights deliver the same amount of energy per second. However, each red light photon is lower in energy than the blue light so there are a greater number of red light photons. The greater number of red light photons causes the photocurrent to be higher for the red light source. Summary on the Photoelectric effect You should know that: Energy has to be supplied to remove electrons from a metal. Surface electrons are the easiest to remove (and need lowest energy). Different metals require differing amounts of energy to release their surface electrons, this energy is called the work function (Ф). A photon can release a surface electron only if it has sufficient energy (hf ≥ Ф). Photon energy is determined by frequency f, so there is a minimum frequency called the threshold frequency fo that can release electrons (hfmin = Ф). Duality: particle or wave? One of the greatest debates in Physics: what is light? Is light a wave or a particle? Isaac Newton : light consists of a stream of particles called corpuscles. Christian Huygens 1655: Light is a wave that propagates through an invisible medium called the luminiferous ether. Thomas Young 1802: Double slit experiment, explained by the principle of wave superposition. Duality: particle or wave? Augustine Fresnel 1815: Explained diffraction. Interference of light waves through oil films. Leon Focault 1850: measured speed of light in air and water. James Clerk Maxwell 1865: light is an EM wave. Heinrich Hertz 1887: light shares properties with radio waves. Albert Einstein 1905: Photoelectric effect; cannot be explained if light is a wave, but can be explained if light acts as a particle. Duality: particle or wave? Contemporary ideas: light can be thought of as being neither a true wave nor particle. However, light does show wave-like and particle-like behaviour but never both at the same time. So the nature of light is essentially schizophrenic, but what about other particles………????? Matter waves Prince Louis de Broglie (1924) established an equation for the relationship between a particle’s momentum and its wavelength. Or in symbol form: Planck’s constant Wavelength = momentum h h λ = = mv p Matter waves Electrons are a form of matter, so these waves are called matter waves; the wavelength of matter waves is sometimes called the de Broglie wavelength. de Broglie concluded that everyday objects are too heavy to observe their wave properties, i.e. their wavelengths are too small to be observed. Example: Calculate the wavelength of a Physics student of mass 50kg running at a speed of 5ms-1 to catch her bus. λ = h mv 6.63 X 10-34 = = 50 X 5 2.65×10-36 m The wave character of the student is so immensely smaller than the student, that there are no experiments we can perform that can probe her wave character. Hence, we never observe students diffracting. Example 2 • When the mass of the object is very small, the wave properties can be detected experimentally. For example, calculate the wavelength of an electron which has a velocity of 1×106 ms-1 h 6.63 × 10-34 -10 m λ = = = 7.29 ×10 9.1× 10-31 x 1×106 mv • The above electron has a similar wavelength to x-rays. • In 1927 the diffraction of electrons was observed experimentally by C. J. Davisson. Wave-Particle duality Most properties of light, such as diffraction, interference and polarisation can be explained by light being considered as a wave. Wave theory cannot explain the photoelectric effect. However, photoelectric effect can be explained by light being considered as a particle. When electrons are fired at graphite or metal targets, a diffraction pattern is produced. Electron diffraction is used as a probe of matter in Physics and as an analytical technique in Chemistry. Electron diffraction X-Ray diffraction Summary of conservation rules In any interaction charge is always conserved Additionally, in