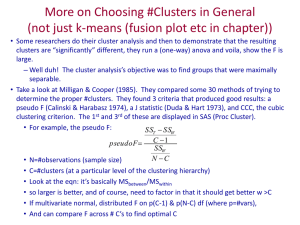
Part 3
advertisement

Atomic & Molecular Clusters
5. Metal Clusters
• Metal clusters have been widely studied – especially
alkali metals, noble metals (Cu, Ag, Au) and transition
metals.
• Cohesive energies are generally quite large (relatively
strong metallic bonding) – significantly higher than for
rare gas or molecular clusters – so they may be studied
in solution (colloidal suspensions), on surfaces or in
inert matrices, as well as in the gas phase.
• A number of models have been introduced to explain
and predict the properties of metal clusters.
The Liquid Drop Model
•
•
•
•
A classical electrostatic model.
Cluster is approximated by a uniform conducting sphere.
Atomic positions and internal electronic structure ignored.
Predictions:
2
3 e
IP W
8 R
2
5 e
EA W
8 R
As 1/R 0 (N )
{IP,EA} W
Energy
IP
W
EA
1/R
{IP(R)W} / eV
{W EA(R)} / eV
M. M. Kappes Chem. Rev. 1988, 88, 369.
1/R / Å
Failures of the Liquid Drop Model
• Deviations from 1/R dependence of IPs and EAs for small
clusters.
• IPs of Hg clusters show a discontinuity due to a sizedependent non-metal metal transition (see later).
• Some transition metals (where ionization involves removal
of tightly bound d electrons, e.g. Fe, Ni) show small
variation of IP/EA with size.
• Magnetic effects (spin-spin interactions) also important for
transition metals.
• LDM does not reproduce fine structure in IP/EA variation
with N (e.g. “even-odd alternation”) or explain the Magic
Numbers in the mass spectra.
• Require a Quantum Mechanical model with discrete
electronic states JELLIUM MODEL.
Mass Spectra and Magic Numbers
• Mass spectra obtained by Knight and co-workers (1983-85),
for alkali metal clusters, showed a number of peaks with high
relative intensities Magic Numbers.
• Magic numbers (and origins) different from rare gas clusters.
The Jellium Model
• Derived from nuclear structure theory.
• Cluster approximated by a sphere with a
uniform positively charged background, filled
with an “electron gas” (valence electrons).
• Valence electrons are delocalized – move in a
smooth, attractive, central, mean field
potential of spherical symmetry.
• Positions of ionic cores are ignored.
• This is justified if:
– electrons are strongly delocalized
– ionic background easily deformed
– molten clusters?
• Works best for monovalent simple metals
– e.g. alkali metals, noble metals (Cu, Ag, Au).
• Unlike the LDM, the jellium model is a
quantum mechanical model
– quantization of electron energy levels due to
boundary conditions imposed by the potential.
• Gives rise to electronic shell structure for
metal clusters with up to several 1000s of
atoms.
Empirical Jellium Models
• Based on effective single-particle potentials (Knight,
Clemenger).
• Solve 1-electron Schrödinger Equation for an
electron in a sphere, under the influence of an
attractive central potential.
ˆ ψ Eψ
H
ˆ T
ˆ V
ˆ
H
• Wavefunction () is separable into radial and angular
parts:
n,,m(r,,) = Rn,(r).Y,m(,)
Solutions
• Wavefunction and energies depend on quantum numbers:
n = 1, 2, 3, …
= 0, 1, 2, …
m = … 0 … +
(no restriction on )
(2 + 1)-degenerate
• Note: the principal quantum number n is different from that used
for atomic orbitals (follows convention of nuclear physics):
nclust = natom -
• Jellium electronic levels (sets of degenerate orbitals) are
labelled, by analogy with atomic orbitals: 1s, 1p, …, 2s, 2p …
• Exact ordering of orbitals depends on the radial form of the
potential.
The Woods-Saxon Potential
• Obtained by fitting to high-level electronic structure
calculations.
U R
U0
exp R R0 1
• W-S potential is a finite well
with rounded sides (intermediate
between 3-D harmonic oscillator
and 3-D square well).
U0 = EF + W
R0 = Ratom.N 1/3
• Ordering of Levels:
1s < 1p < 1d < 2s < 1f < 2p < 1g
…
• Level Closings
(no. of electrons)
2 8 18 20 34 40 58 …
Energy
58
1g
40
2p
34
1f
20
18
8
2
2s
1d
1p
1s
Interpreting Mass Spectra of Metal Clusters
1. Low Energy Ionization
MN
h
M N+
+ e
•
Magic numbers (intense peaks in MS) due
to stable electron counts (filled jellium
levels) of neutral clusters (MN).
•
N* = 8, 20, 40, 58 …
2. High Energy Ionization
highly electronically
excited
MN
e
M N+
+ e
high E h
evaporation
of M atoms
M X+
+ (N-X) M
•
Magic numbers due to stability of cationic
clusters (MX+):
N* = 9, 21, 41, 59 …
•
Note: Na8, Na9+ both have 8 electrons.
Breakdown of the Spherical Jellium Model
• Fine structure is observed in the MS, IPs,
EAs, polarizabilities etc., for even-electron
counts other than those predicted by the
(spherical) jellium model.
• This is evidence for non-degenerate
electronic sub-levels, which cannot be
explained by the spherical jellium model.
• Need to extend the model.
The Ellipsoidal Shell Model
• Modification to spherical
jellium model, introduced
by Clemenger (1985).
• Potential = a perturbed
3-D harmonic oscillator
– analogous to Nilsson’s
model (1955) for nuclear
structure.
• Ellipsoidal (“spheroidal”)
distortion of cluster.
Iz = moment of inertia
about z-axis etc.
• Lowering of symmetry loss of (2+1)-fold degeneracy
of each jellium level (n).
• m degeneracy is maintained in ellipsoidal (spheroidal)
symmetry.
• Oblate Spheroid E as |m|
> ½-filled shell
• Prolate Spheroid E as |m|
< ½-filled shell
1
(np)4
1
=1
(np)2
Variation of Na Cluster Shape with Size
Comparison of structures of Na and Ar clusters
Beyond the Jellium Model
• Martin and co-workers (1991)*
measured MS of NaN clusters (N 25,000).
• Observed two series of
periodic intensity variations:
period N1/3.
N1/3
* T. P. Martin et al., J. Phys. Chem. 1991, 95, 6421.
Electronic Shells (N < 2000)
• Electronic shells form due to bunching together of
jellium levels.
• Electronic shells = sets of nearly degenerate
jellium levels.
• For larger metal clusters, electronic effects
are relatively unimportant because electronic
shells merge to form quasi-continuous bands
(bulk-like band structure).
jellium
levels
N
electronic
shells
band
structure
Geometric Shells (N > 2000)
• Geometric shells correspond to complete
concentric polyhedral shells of atoms – as for rare
gas clusters.
• Stability due to minimization of surface energy.
• Alkali Metal Clusters – magic numbers are
consistent with filling K geometric shells:
N * K 1
10 k
K
k 1
2
2
1
3
10 K
3
15 K
2
11K 3
Examples of Geometric Shells
rhombic
dodecahedron (bcc)
icosahedron
truncated
octahedron (fcc)
• Similar magic numbers have been observed for Ca
clusters with up to 5000 atoms.*
CaN+
N
• MS magic numbers and fine structure (due to partial
geometric shell formation) indicate that alkali metal
clusters (with N > 2000) and Ca clusters have
icosahedral shell structure.
* T. P. Martin Physics Reports 1996, 273, 199.
• Al and In clusters form octahedral shell structures
(fragments of fcc packing).
InN+
• Geometric shell structure has also been found for
many transition metal clusters (e.g. Co, Ni).
• Electronic shell effects are relatively unimportant for
TMs with unfilled d-orbitals as the onset of band
structure occurs for quite low N.
Microscopy Studies of Metal Clusters
• A number of microscopy techniques can be applied to
study metal clusters:
– Electron Microscopy (TEM, SEM)
– Scanning Tunnelling Microscopy (STM)
– Atomic Force Microscopy (AFM)
• Clusters must be immobilized on a substrate (e.g.
graphite, amorphous-C, MgO, SiO2 – depending on
the type of measurement).
• Clusters are often passivated by surfactant (ligand)
molecules.
• Cluster-surface and cluster-ligand interaction may
affect cluster structure (for small clusters).
Electron Micrographs of Ag and Au Particles
15 nm
4 nm
Single Ag decahedron
Multiply-twinned Ag fcc particle
3 nm
7 nm
Intergrowth of 2 Au
icosahedra
Truncated octahedral
Au fcc particle
Marks decahedral
Au particle
Energetics of pure Ag clusters
Mackay Icosahedra
Quasi-spherical shape.
Close-packed surface but
strong internal strain.
Maximizes the number of
NN bonds
favourable at small sizes
Marks Decahedra
Intermediate behaviour.
Favourable at
Intermediate sizes
Fcc Polyhedra
Non-spherical shape
but no internal strain.
Fewer NN bonds.
Favourable at large sizes
Insulator-Metal Transition in Hg Clusters
• Rademann and Hensel (1987) measured IPs of Hg clusters as a
function of size, N.
• Explained in terms of a gradual transition:
Insulating Semi-Conducting Metallic
in the region N ~ 13-70.
• Consistent with spectroscopy
and theoretical calculations.
Theory of Bonding in Hg Clusters
• The free Hg atom has a closed shell: (6s)2(6p)0
– Small clusters are insulating “van der Waals clusters”
– held together by dispersion forces.
• As the cluster gets larger, the 6s and 6p levels
broaden into bands (with widths Ws and Wp)
– W as N
• Insulator Metal Transition occurs when the 6s and
6p bands overlap.
• Before band overlap (intermediate N), the band gap
(sp) may be comparable to the thermal energy (kT)
– semi-conductor clusters
– s-p hybridization occurs covalent bonding
Size-dependent Electronic
Structures of Hg Clusters
Wp
6p
sp kT
sp
6s
Atom
Ws
Insulating
van der Waals
Clusters
E
sp
Metallic Clusters
& Bulk Metal
F