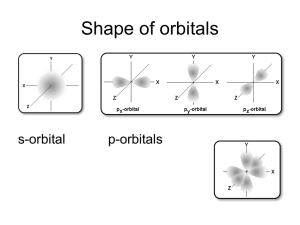
electron energies
advertisement

2. Ionization - Electron impact ionization & Photo ionization - WS2011/12 2.1 Overview 1. Atomic processes in plasma 2. Rate equation Example EBIT, Charge Exchange, Radiative Recombination 3. Electron impact ionization Ionization mechanism: Multistep ionization, Ionization factor, approximation for cross section (Lotz formula), Carlson correction, optimal electron energy, application (electron target) 4. Photo ionization Basics, cross section, application (RILIS) 5. Summary WS2011/12 2.2 2.1. Overview: Atomic processes in a plasma In most ion sources, ions are produced in a plasma. The basic atomic processes (selection) in plasmas are: collisions with electrons Impact Ionization Z A Three-Body-Recombination (TBR) Z 1 e A Impact excitation AZ e Photo ionization e' e' ' Impact disexcitation Z A e' collisions with photons AZ h Photo absorption Z A Non-radiative transition Radiative Recombination (RR) AZ h AZ 1 e Excitation AZ+: Atom of species A with charge state Z e’ : electron changed energy Line spectrum Spontaneous emission A Z Bremsstrahlung Z h e A e' Continuous spectrum The electron changes from one free state to another free state with lower energy. WS2011/12 2.3 2.2. Rate equation From these multiple processes arise the dynamic balance quantities: • Distribution of the abundance of all charge states Z (=0...Zmax), Ionization equilibrium • Number of emitted and absorbed photons per time interval, Radiative equilibrium The density of the particle species are determined from so-called rate equations: dn souces sinks dt Example: impact ionization: dnz 1 ne nz v e z z 1 ne nz 1 z 1,TBR dt ß z+1, TBR : rate coefficient for Three-Body-Recombination (TBR) The rate coefficients often not be calculated with sufficient precision; experimental data are only available to a limited extent. Therefore one tries to obtain data from thermo dynamical equilibrium. With decreasing electron density the TBR drops, so that the impact ionization is not in equilibrium with the TBR anymore. The RR rate also decreases but not as strong. With decreasing ne also the photo ionization becomes unlikely. As result the impact ionization and the RR-process dominate. The photons leave the plasma without being re-absorbed. WS2011/12 2.4 Example EBIT: dni ion RR RR chex chex coll n e e iion 1 i n i 1 i i 1 i i 1 n i i 1 i n i 1 n o ion i i 1 n i i 1 i n i 1 i dt ion : averaged thermal ion velocity ion 2kTion Mion : cross sections for impact ionization, RR and charge exchange by collisions with residual gas icoll : collision rate for all Coulomb collisions of ions with charge state i UW 16 ARGON 0 log 80 60 : depth of the electrostatic potential (radial and axial), kTion : thermal energy of the ions heated by Coulomb collisions nee : electron density and velocity no % ieU w exp kTion ni ieU w kTion 8 40 20 : neutral particle density The solutions of the linear system of equations provides the charge state distribution in dependence je on the ionization factor 16 % 0 ARGON 80 -3 -2 -1 log(ni/nma x) 0 1 2 log(J*TAU) ARGON -1 16 TOP: 60 Ionization of pulsed injected argon gas by an electron beam with an energy of 3.9 keV, which inhibits the ionization of the charge 8 state Ar17+ (EBIS-Mode 40 with breeding at the shell closure). BOTTOM: -3 8 -4 20 Capture and ionization for the case of continuously injected argon atoms and 6 keV electron beam energy (EBIT-Mode without RR -3 residual -2 gas).-1 0 1 2 and charge exchange with log(J*TAU) WS2011/12 -2 -3 -2 -1 0 1 2 3 log(J*TAU) 2.5 Charge exchange: For the approximation of the charge exchange cross section, commonly the equation by Müller and Salzborn is used: 12 1.17 2.76 2 iex 1 . 43 10 i E cm i 1 ion,gas Therein i describes the charge state of the highly charged ion and Eion, gas is the ionization potential of the gas atoms interacting with the ion. Example: Reachable charge states depending on the current density and the pressure -3 10 -6 Pressure (mbar) 10 100 A/cm 2 -9 10 10 A/cm 2 -12 10 0 20 40 60 80 Pb Charge states WS2011/12 2.6 Radiative Recombination (RR): For the approximation of the RR cross sections the semi-classical expression by Kim und Pratt is used. Their formula bases on the first theoretical description of the Radiative Recombination by Kramers (1923): with with: • the effective nuclear charge potential by the bound electrons to take into account the shielding of the nuclear • the effective main quantum number to include the capture into states with different angular momentums Ry: a: w0 Rydberg energy 13,6 eV fine structure constant ratio between the numbers of occupied and unoccupied states Z: nuclear charge q: charge state n: main quantum number (le)r : reduced Compton-wavelength E: electron energy WS2011/12 2.7 2.3. Electron impact ionization The electron impact ionization is the most fundamental ionization process for the operation of ion sources. Why? • The cross section for the impact ionization is by orders of magnitudes higher than the cross section for the photo ionization. • The cross section depends on the mass of the colliding particle. Since the energy transfer of a heavy particle is lower, a proton needs for an identical ionization probability an ionization energy three orders of magnitudes higher than an electron WS2011/12 2.8 Mechanism for the impact ionization There are two different possibilities to produce multiple charged ions: • in a collision where many electrons are removed from the ion (double-ionization, triple-ionization, …) • multistep or successive ionization, where only one electron is removed per collision and high charge states are produced in different collisions Electron impact Double ionization Cross section Cross section Electron impact Single ionization electron energy electron energy For energetic reasons the ionization releasing only one electron from the atomic shell is the most probable process. To produce highly charged ions the kinetic energy of the projectile electrons has to be at least equivalent to the n-th ionization potential. WS2011/12 2.9 Ionization energies up to 100 keV (U91+ → U92+) Ionisation energies by C. Moore 1E+6 eV 1E+5 1E+4 1E+3 1E+2 1E+1 1E+0 0 10 20 30 40 50 OXGAS2.XLS R. Becker, 31.07.99 60 70 80 90 100 Z → shell structure of the atomic shells is clearly visible WS2011/12 2.10 Successive ionization: The probability for removing one electron and changing the charge state of the ion from q q+1 is determined by the cross section qq+1 [cm2]. Are the cross sections for the successive ionization known, the average ionization time of the ions with charge state q can be approximated from the collision frequency: q q 1 ne nq ve q q 1 1 m3s The time between to ionizing collisions is qq1 nq qq1 1 neve qq1 e je qq1 This applies to electrons with a defined kinetic energy. je q q 1 e q q 1 The average ionization time in the charge state q depends only on the cross section and the current density. This expression is called IONISATION FACTOR. (Sometimes it is defined as only the inverse cross section!) WS2011/12 Principle of electron impact ionization 2.11 The average time necessary to reach the charge state q is therewith: q 1 e q 1 1 q i i 1 je i 0 i i 1 i 0 Example on the right side: The ionization factor je* for different charge states of Xe depending on the electron energy. Approximation of the cross section and the ionization time for the production of bare ions from H-like ions using the Mosley’s law for X-ray frequencies emitted in transitions from the continuum to the K-shell: Eik (Z ) 13.6 Z [eV ] z 1z 4.5 10 2 E e Eik (Z ) j 1 z 1z e z1z e z1z 4 e eZ 4 Z je z 1z z 1z 9 1017 5 14 ln e 9 1017 2 4 e 13.6 Z Z4 wherein Examples: Argon can be ionized by 10 keV electrons, ions of the heavy elements by up to 100 keV electrons and Uranium by 150 keV electrons. The resulting values for the ionization energy, cross section and ionization factor are summarized in the table on the left side. WS2011/12 2.12 Approximation of the cross section for the electron impact ionization Approximation of the cross section in quantum-mechanical calculations done by Bethe (1930) using the Born Approximation: Scattering of a matter wave at a central potential V(r) for Ekin >> Eion (perturbation theory). All electrons in an atom or ion contribute with their individual ei to the total cross section , as long as the kinetic energy Ekin of the projectile is larger then the ionization energy Pi of these electrons. N N q q 1 i qi ei i 1 N = number of subshells i 1 All qi electrons of the subshell contribute to the i of the shell. The cross section for the ionization of the (n, l) - shell results from integration of the transition probabilities over all states n’, l’ k und the integration over the collision vector 2 q M (v v ' ) h with v before and v’ after the collision. One receives inl const Znl WS2011/12 E 1 ln kin EkinEnl Enl Bethe et al., Ann. Physik 5 (1930) 325 2.13 For practical reasons the semi-empirical formula developed by Lotz 1967 for the energy dependence of the cross sections for the elements from H to Ca and for energies < 10 keV is commonly used. The error is given by maximal 10%. The Lotz formula for the case of high ionization energies Ekin >> Pi is: Ekin ln N Pi 14 q q 1 4.5 10 Ekin Pi i 1 cm 2 Pi = Enl , N-subshells This expressions is mostly used in calculations of the charge state distribution. For E Pi and with follows E 1 x kin Pi x2 (x 0) , and with ln (1 x ) x 2 0 1 4.5 1014 ( EPkin1 1) P12 for x << 1 ~ Ekin This means the cross section goes linear with E Pi to zero. Examples for the dependence of the cross sections on the energy are shown for the case of He. The higher the initial charge state, the smaller is the cross section. Moreover the cross section is higher for atoms in an excited state. WS2011/12 2.14 Single-ionisation: He + e → He+ + 2e 10-17 Multi-ionisation: He + e → He2+ + 3e 1019 WS2011/12 2.15 Ionization of the excited state 10-16 WS2011/12 He (2s) + e → He+ + 2e Ionization of singly charged He 10-18 He+ + e → He2+ + 2e 2.16 Carlson-Correction for ionization energies: The ionization energies Pq,i for ions with different charge states q, which does not describe the weakest bound electron are sometimes difficult to find in literature. They can be approximated with the CarlsonCorrection. The ionization energy Pq,i is calculated from the ionization energy Wi(q) of the ion with charge state q and the atomic binding energies of the electrons as follows: Pi E0i Wi (q) E0q E0i E0q : binding energy of an electron in the i-th shell of an atom : atomic binding energy of the electron, which is the weakest bound electron in the ion of the charge state q Wi(q) : ionization energy of the ion (describes always the weakest bound electron) weakest bound electron q (ionization energy is known) E0q-E0i Ion: Aq+ WS2011/12 inner electron i, to be removed 2.17 Optimal electron energy Because (E) has a maximum at a certain energy, the ionization factor je* has a minimum there. Basically the cross section for the last electron, which is removed, determines the ionisation time. The optimal energy is given by d z z 1 dE ln Ekin Pi d 14 0 4.5 10 Ekin Pi i 1 dE N E 1 0 Emax 1 ln 2 i 1 Pi E Pi N N 1 ln Pi P exp i 1 N i 1P i i 1 N ln Pi e exp i 1 Pi N 1P i i 1 For the optimal energy of the last electron, which is removed, follows: E max ln Pz P e exp z 1 Pz eP z Therewith the optimal energy is nearly e-times the ionization energy of the last electron that is removed from the ion with the charge state z. WS2011/12 2.18 electron energy Ionization factor and optimal electron energy ionization factor WS2011/12 2.19 Application of the electron impact ionization for the production of ions Slow electrons Fast ions Stripper foil MI ++++++ Fast electrons ECR plasma MI +++ Slow ions +++ +++ Fast electrons +++ +++ EBIS beam Slow ions MI • Plasma confinement by magnetic fields of different structure (cusp, magnetic mirror, …) (see next lectures) • The efficiency of the electrons in the plasma and the charge state can be increased by using the electron several times, by: - reflection of the electrons, e.g. reflection discharge or penning discharge - magnetic confinement, e.g. by magnetic mirrors WS2011/12 2.20 Further application of electron impact ionization Example: electron target Investigation of electron-ion-collisions: • Investigation of electron impact ionization • Investigation of recombination processes • Investigation of excitation processes Measurement of rate coefficients R R a ne r ni r d 3r An+ and therewith cross sections: a v r v r v r f v r d 3v r Important is the overlap of ion and electron beam : cross section ne: electron density ni: ion density vr: collision velocity / relative velocity f(vr): distribution function of the relative velocity WS2011/12 An+ vi vr ve 2.21 Transversal electron target Longitudinal electron target • „Merged beams“. • also used as cooler for ion beams • 2-2.5m long interaction region • “crossed beams technique” • better energy resolution then gas target • 10-15 cm long interaction region • spectroscopic access is possible • electrostatic focusing BUT: • limited access for spectroscopy BUT: • lower interaction rate as longitudinal electron target or gas target 0.061A/cm, R=8.19 mesh units, J=0.2 A/cm2 WS2011/12 2.22 2.4. Photo ionization The reaction is: A h A e Atoms of a gas can be ionized by an intensive beam of photons with the adequate energy (photo ionization). Therefore the photon energy has to be h e q,i The energy of a photo electron is: Cross section p: . 1 2 mvmax h e q , i 2 Z 4 5 p ( w ) 7 / 2 • p has a strong dependence on the photon energy and the nuclear charge Z: • For a given atomic shell the cross section p is the largest close to threshold, meaning where the photon energy reaches the ionization energy I (resonance/ threshold behavior): max : w IK , IL , IM pn : cross section for RR into the K-shell n: main quantum number cross section (barn) • For high photon energies ω I K the ionization of the s-orbital is most probable and the K-shell ionization delivers the dominant contribution: 1 1 p n 3 K → p p 3 1.2021 p K K n n 1 n 1E7 1000000 100000 10000 1000 100 10 0,1 • Description of p as time-inverse effect to the radiative recombination by the 2 w Milne-formula: gq 1 RR gq P 2mec 2E WS2011/12 1 10 100 photon energy (keV) with g: statistical weights 2.23 Application of photo ionization for the production of ions 1 eV l = 1.24 mm (IR-radiation), 5 eV l = 248 nm (close UV) For direct ionization photons from the UV – x-ray region are necessary. RILIS (Resonant ionization laser ion source) Ionization via resonant excitation with three laser beams of frequencies f1 – f3, as shown in the following scheme. WS2011/12 2.24 In reality there a many more effects to be takeen into account: • Excitation into auto-ionizing state (AIS) with typical lifetimes of 10-15 – 10-10 s • Excitation in Rydberg-states n = n* Lifetime Binding energy 0 (n* )3 M Z2 E R M me (n* )2 R = Rydberg constant Radius of Rydberg atoms r a0(n*)2 Orders of magnitudes for the cross sections: • non-resonant (direct ionization): = 10-19 – 10-17 cm2 • AIS: = 1.6 x 10-14 cm2 • Rydberg states: ~ 10-14 cm2 WS2011/12 Excitation schemes used for resonant laser ionization 2.25 A prominent example for a RILIS is at ISOLDE/CERN (see picture). Advantages of a RILIS: • high selectivity • separation of surface-ionizing contaminations by adjusting the temperature of the cavity • high efficiency WS2011/12 2.26 2.5. Summary: Ion production Processes increasing the charge state Processes decreasing the charge state • Ionization - single-ionization - double-ionization • Recombination - radiative recombination The production of higher charge states is a successive process The cross section is larger for lower electron temperatures - dielectronic recombination (resonant process) The ionization has energy threshold → higher charge states need higher projectile energies (electron energies) • Charge exchange (for low charge states) • Charge exchange (for high charge states) depending on the neutral particle density (residual gas) cross section are larger for higher charge states WS2011/12 2.27 10. Spouses never need to worry about what their ion source physicist husband or wife is up to when they still aren’t home by midnight...they know with great confidence that they’re just at the lab changing a source! 9. Between – – – – – – – the International Conference on Ion Sources, the Workshop on Ion Source Issues Relevant to a Pulsed Spallation Neutron Source, the ECR Ion Source Workshop, The International Conference on Negative Ions, Beams and Sources, the International Symposium on the Production and Neutralization of Negative Ions and Beams, the Workshop on Negative Ion Formation and Beam Handling, the International Conference on Sources of Highly Charged Ions, the ion source community has the greatest number of conferences and workshops of any scientific discipline measured per linear inch of subject matter 8. Endless hours can be devoted to the important philosophical meaning of “pi” as in “the output emittance is 0.2 pi-mm-mrad” 7. Believe it or not, sometimes the SNS ion source antenna picks up free Satellite Television! 6. High-voltage, hydrogen gas, antennas and power supplies: it’s every little kids dream! 5. In what other field can you acquire a Ph.D., and then spend your professional life practicing the occult? 4. When the phone rings during dinner time, you always know who it is: no its not a tele-marketer, it just the lab...the ion source went down! 3. In what other field could you advertise a workshop on “RF-driven, multicusp, Cesium-enhanced H-sources for the SNS”, and have 50 people actually show up? 2. Job security...when was the last time you heard a manager say, “the ion source is running so well, we’re going to let the entire group go.” 1. It’s a subject in which anyone can make a contribution, and everyone has an opinion! WS2011/12 Courtesy of Stuart Henderson/ORNL 2.28