bonding - Chemistry
advertisement

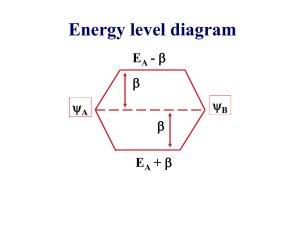
Molecular Orbital Theory and Charge Transfer Excitations Chemistry 123 Spring 2008 Dr. Woodward Molecular Orbital Diagram H2 H 1s Orbital Bonding Molecular Orbital (Orbitals interfere constructively) H 1s Orbital Energy Antibonding Molecular Orbital (Orbitals interfere destructively) Orbital overlap – Constructive Interference B o n d in g O v e rla p 1 s A to m 1 Constructive Interference W a v e fu n c tio n ( ) 1 s A to m 2 Between the nuclei the wavefunctions add together. Electron density maximized in the internuclear region. A to m (2 ) A to m (1 ) This type of overlap leads to formation of a covalent bond. -4 -3 -2 -1 0 1 2 3 z (A n g s tro m s ) + = 4 Orbital overlap – Destructive Interference A n tib o n d in g O v e rla p Destructive Interference 1 s A to m 1 1 s A to m 2 W a v e fu n c tio n ( ) Between the nuclei the wavefunctions cancel each other out. Electron density pushed away from internuclear region. A to m (2 ) A to m (1 ) This type of overlap works against formation of a covalent bond. -4 -3 -2 -1 0 1 2 3 z (A n g s tro m s ) + = 4 Pi (π) Bonding Sigma (σ) Bonding side on overlap head on overlap + Atom 1 pz orbital = Atom 2 pz orbital + Bonding pi molecular orbital Constructive Interference + Atom 1 pz orbital Atom 1 py orbital Atom 2 py orbital + Antibonding pi molecular orbital Destructive Interference Bonding sigma molecular orbital Constructive Interference = Atom 2 pz orbital = Atom 1 py orbital = Atom 2 py orbital Bonding sigma molecular orbital Destructive Interference Principles of MO Theory 1. Conservation of Orbitals: The number of Molecular Orbitals is equal to the number of Atomic Orbitals. 2. Conservation of Electrons: The number of electrons occupying the molecular orbitals is equal to the sum of the valence electrons on the constituent atoms. 3. Pauli Exclusion Principle: Each MO can hold two electrons of opposite spin. 4. Hunds Rule: When orbitals are degenerate (at the same energy) all electron spins are the same direction (up) until we have to start putting two electrons in the same orbital. 5. Principle of Orbital Mixing: The splitting between bonding and antibonding MO’s decreases as: a. The spatial overlap decreases (due to orientation of the orbitals, interatomic distance, or size of orbitals) b. The orbital electronegativities become different Cr3+ 5 d-orbitals on Cr (Cr3+ = d3 ion) 3 electrons in the d-orbitals [Cr(NH3)6]3+ Octahedron :NH3 : N H H H 6 Ligand Orbitals Nitrogen lone pairs (all containing 2 e-) Only sigma interactions are allowed [Cr(NH3)6]3+ Antibonding (s*) Metal-Ligand MO’s eg orbitals (dz2, dx2-y2) D = Crystal Field Splitting Energy t2g orbitals (dxz, dyz, dxy) Energy Metal (Cr) d-orbitals Nonbonding Metal d MO’s Nonbonding Ligand MO’s Bonding (s) Metal-Ligand MO’s Ligand (N) lone-pair orbitals Absorption Spectra Cr3+ Solutions Antibonding (s*) Metal-Ligand MO’s 1 .4 [C r(H 2 O )6 ]3 + 1 .2 [C r(O H )4 (H 2 O )2 ]1 - a b s o rb a n c e 1 .0 [C rO 4 ]2 - Doct 0 .8 0 .6 Nonbonding Metal d MO’s 0 .4 0 .2 0 .0 250 350 450 550 w a ve le n g th (n m ) 650 750 850 [CrO4]2- t2 orbitals (more antibonding) e orbitals (antibonding) CT Energy Metal (Cr) d-orbitals Nonbonding Oxygen 2p MO’s e orbitals (bonding) t2 orbitals (bonding) 12 Oxygen 2p orbitals (4 oxygens x 3 p orbitals) Absorption Spectra CrO42- Solutions Antibonding Cr 3d orbitals 1 .4 [C r(H 2 O )6 ]3 + 1 .2 [C r(O H )4 (H 2 O )2 ]1 - a b s o rb a n c e 1 .0 [C rO 4 ]2 - 0 .8 CT1 0 .6 0 .4 0 .2 0 .0 250 350 450 550 w a ve le n g th (n m ) 650 750 850 Nonbonding Oxygen 2p MO’s Absorption Spectra CrO42- Solutions Antibonding Cr 3d orbitals 1 .4 [C r(H 2 O )6 ]3 + 1 .2 [C r(O H )4 (H 2 O )2 ]1 - a b s o rb a n c e 1 .0 [C rO 4 ]2 - CT2 0 .8 0 .6 0 .4 0 .2 0 .0 250 350 450 550 w a ve le n g th (n m ) 650 750 850 Nonbonding Oxygen 2p MO’s Charge Transfer Salts, ACrO4 The absorbance of SrCrO4 is similar to a concentrated solution of CrO42- ions. Charge Transfer Excitations and Periodic Trends We can expect charge transfer transitions when we have a d0 cation in a high oxidation state. How does the charge transfer change as we move around the periodic table? CrO42- vs. MnO42- Antibonding (e) Cr dx2-y2, dz2 CT Antibonding (e) Mn dx2-y2, dz2 CT Nonbonding O 2p Nonbonding O 2p [CrO4]2- [MnO4]- As the cation oxidation state increases [i.e. Cr(VI) Mn(VII)] d-orbitals become more electronegative (lower in energy) CT Energy Gap decreases Absorption shifts to longer wavelengths SrMoO4 – SrCrO4 Series S rC rO 4 - S rM o O 4 K ris te n 100 90 a b s o rb a n c e 80 2- 60 S rC rO 4 [C rO 4 ] R e fle c ta n c e 70 50 S rC r0 .9 M o 0 .1 O 4 S rC r0 .8 M o 0 .2 O 4 40 S rC r0 .5 M o 0 .5 O 4 S rC r0 .2 M o 0 .8 O 4 30 S rC r0 .1 M o 0 .9 O 4 S rM o O 4 20 C rO 4 (2 -) 10 0 SrMoO4 250 350 450 550 W a ve le n g th (n m ) 650 750 SrCrO4 Orbital Radii – Group 6 Cr 4s r = 1.63 Å Mo 5s Cr 3d r = 0.46 Å Mo 4d r = 1.75 Å r = 0.73 Å W 6s W 5d r = 1.65 Å r = 0.78 Å The d orbitals are always much smaller than the s and p, but the 3d orbitals are particularly small Antibonding (e) Cr dx2-y2, dz2 CT Nonbonding O 2p [CrO4]2- Antibonding (e) Mo dx2-y2, dz2 CT Nonbonding O 2p [MoO4]2- Mo 4d orbitals are larger than the Cr 3d orbitals d-orbitals interact more with O 2p orbitals – more antibonding CT Energy Gap increases Absorption shifts to shorter wavelengths 2nd & 3rd Row Transition Metals eg (s*) 2nd and 3rd row transition metals •d-orbitals are larger •Metal-ligand antibonding interactions are stronger •eg (s*) orbitals are more antibonding •Low spin configurations are always observed [Co(H2O)6]3+ D = 2.25 eV [Rh(H2O)6]3+ D = 4.23 eV