Document
advertisement

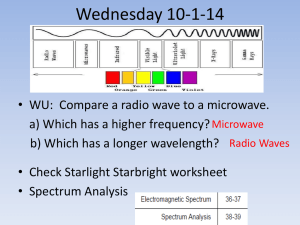
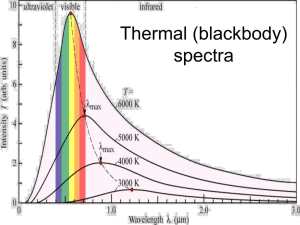
Chapter 7 Starlight and Atoms Guidepost Some chapters in textbooks do little more than present facts. The chapters in this book attempt to present astronomy as organized understanding. But this chapter is special. It presents us with a tool. The interaction of light with matter gives astronomers clues about the nature of the heavens, but the clues are meaningless unless astronomers understand how atoms leave their traces on starlight. Thus, we dedicate an entire chapter to understanding how atoms interact with light. This chapter marks a transition in the way we look at nature. Earlier chapters described what we see with our eyes and explained those observations using models and theories. With this chapter, we turn to modern astrophysics, the application of physics to the study of Guidepost (continued) the sky. Now we can search out secrets of the stars that lie beyond the grasp of our eyes. If this chapter presents us with a tool, then we should use it immediately. The next chapter will apply our new tool to understanding the sun. Outline I. Starlight A. Temperature and Heat B. The Origin of Starlight C. Two Radiation Laws D. The Color Index II. Atoms A. A Model Atom B. Different Kinds of Atoms C. Electron Shells III. The Interaction of Light and Matter A. The Excitation of Atoms B. The Formation of a Spectrum Outline (continued) IV. Stellar Spectra A. The Balmer Thermometer B. Spectral Classification C. The Composition of the Stars D. The Doppler Effect E. Calculating the Doppler Velocity F. The Shapes of Spectral Lines The Amazing Power of Starlight Just by analyzing the light received from a star, astronomers can retrieve information about a star’s 1. Total energy output 2. Surface temperature 3. Radius 4. Chemical composition 5. Velocity relative to Earth 6. Rotation period Color and Temperature Stars appear in different colors: • blue (like Rigel) Orion Betelgeuse • green / yellow (like our sun) • red (like Betelgeuse). These colors tell us about the star’s temperature. Rigel Black Body Radiation The light from a star is mostly ultraviolent (sunburn!), visual (roygbiv!), and infrared (heat). The star’s light is nearly a black body spectrum. Two laws of blackbodies: 1. The hotter an object is, the more luminous it is 2. The hotter the object is the more the black body spectrum shifts towards shorter wavelengths. The Color Index The color of a star is measured by comparing its brightness in the blue (B) band and the visual (V) band. A color index measures both B-band & V-band magnitude, and takes the difference (B minus V, or simply B – V). The hotter (more blue) a star, the smaller the color index: Blue stars: B – V = -0.4 50,000 degrees Kelvin! Red stars: B – V = +2.0 2,000 degrees Kelvin B band V band Light and Matter Spectra of stars are more complicated than pure blackbody spectra because of characteristic absorption lines. To understand those lines, we need to understand atomic structure and the interactions between light and atoms. Atomic Structure video clip video clip • An atom consists of an atomic nucleus The nucleus has positive protons and neutral neutrons, and a cloud of electrons surrounding it. • Almost all of the mass is contained in the nucleus, while almost all of the space is occupied by the electron cloud. • The nucleus is so dense that a teaspoon of it would weigh about 2 billion tons!! Different Kinds of Atoms The kind of atom depends on the number of protons in the nucleus: • Hydrogen (H), has one proton (and 1 electron). • Helium (He), has 2 protons (and 2 neutrons & 2 electrons). Atoms can collide and bond into molecules, but only in “cool” stars. If an atom loses or gains electrons, it is called an ion. Different numbers of neutrons = different isotopes Electron Orbits • Electron orbits in the electron cloud are restricted to very specific radii and energies. r3, E3 r2, E2 r1, E1 • These characteristic electron energies are different for each individual element. Atomic Transitions • An electron can be kicked into a higher orbit in a collision or when it absorbs a photon with the right energy/wavelen • gth. The photon is absorbed, and the electron is in an excited state • When the electron returns to the ground state it will emit a photon. video clip Ephoton = E3 – E1 Ephoton = E4 – E1 • The spectrum of a star forms as light passes outward through gases near its surface Kirchhoff’s Laws of Radiation (1) 1. A solid, liquid, or dense gas excited to emit light will radiate at all wavelengths and thus produce a continuous spectrum. Kirchhoff’s Laws of Radiation (2) 2. A low-density gas excited to emit light will do so at specific wavelengths and thus produce an emission spectrum. Light excites electrons in atoms to higher energy states Transition back to lower states emits light at specific wavelengths Kirchhoff’s Laws of Radiation (3) 3. If light comprising a continuous spectrum passes through a cool, low-density gas, the result will be an absorption spectrum. Light excites electrons in atoms to higher energy states Wavelengths of light corresponding to the transition energies are absorbed from the continuous spectrum. The Spectra of Stars video clip Inner, dense layers of a star produce a continuous (blackbody) spectrum. Cooler surface layers absorb light at specific frequencies. Therefore, spectra of stars are absorption spectra. Kirchhoff’s Laws (SLIDESHOW MODE ONLY) Analyzing Absorption Spectra • Each element produces a specific set of absorption and emission lines. • Comparing the relative strengths of these sets of lines, we can study the gases from stars. Where to start? With the most abundant elements in the universe! Lines of Hydrogen Most prominent lines in many astronomical objects are Balmer lines of hydrogen Observations of the H-Alpha Line Emission nebula, dominated by the red hydrogen alpha (H ) line. Absorption Spectrum Dominated by Balmer Lines Modern spectra are usually recorded digitally and represented as plots of intensity vs. wavelength The Balmer Thermometer Balmer line strength tells us star temperature In medium temp. stars (10,000K), Balmer lines are strong In cooler stars (<<10,000 K) almost all hydrogen atoms are in the ground state, so Balmer lines are weak. In hotter stars (>> 10,000K) most hydrogen atoms are ionized, so Balmer lines are weak Measuring the Temperatures of Stars A star’s surface temperature is measured by comparing many lines A very hot star (40,000K) has weak Balmer lines and strong ionized helium lines. A very cool star (3,000K) has weak Balmer lines and strong titanium oxide lines. Spectral Classification of Stars (1) Temperature Each spectral class divides Different types of stars show different into 10 subclasses (0 to 9) characteristic sets of absorption lines. Spectral Classification of Stars (2) Mnemonics to remember the spectral sequence: Oh Oh Only Be Boy, Bad A An Astronomers Fine F Forget Girl/Guy Grade Generally Kiss Kills Known Me Me Mnemonics Stellar Spectra F G K M Surface temperature O B A The Composition of Stars From the relative strength of absorption lines (carefully accounting for their temperature dependence), one can infer the composition of stars. The Doppler Effect higher pitch lower pitch Blue Shift (to higher frequencies) Red Shift (to lower frequencies) Waves from a source are shifted in observed frequency when the source/observer move toward each other. Light of different frequency is seen as a different color. Increase in observed frequency is called a blue shift. Decrease in observed frequency is called a red shift. Doppler Shift If a star is moving toward Earth, the lines in its spectrum are shifted slightly toward shorter wavelength (higher frequency). This shifts the absorption lines toward the blue end of the spectrum, so it’s called a blue shift. If a star is moving away from Earth, the lines in its spectrum are shifted slightly toward the longer wavelength (lower frequency). This creates a red shift in the absorption spectrum. New Terms temperature Kelvin temperature scale absolute zero thermal energy electron black body radiation wavelength of maximum intensity (λmax) color index nucleus proton neutron isotope ionization ion molecule Coulomb force binding energy quantum mechanics permitted orbit energy level excited atom ground state continuous spectrum absorption spectrum (dark-line spectrum) absorption line emission spectrum (brightline spectrum) emission line Kirchhoff’s laws transition Lyman series Balmer series Paschen series spectral class or type