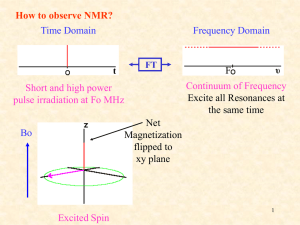
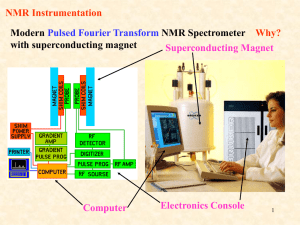
NMR Spectroscopy
advertisement

NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
• T1 relaxation: Remember that spin–lattice, or longitudinal, relaxation
returns the system to equilibrium along the z axis, with time constant T1 and
rate constant R1 = (1/T1)
From: Hornack, J.P., http://www.cis.rit.edu/htbooks/nmr/
• T2 relaxation is due primarily to scalar coupling (J-coupling) by the Fermi
contact mechanism. T2 relaxation is in the component of relaxation in the x-
y plane.
• By definition T2 < T1
CHEM 430 – NMR Spectroscopy
2
NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
• T1 relaxation: The first T1 is observed when placing the sample in the
magnet! The initial bulk magnetization has a time constant of T1!
© Copyright Hans J. Reich 2013
• Knowledge of T1 is important. When using FT-NMR techniques, the time
between pulses must allow for full relaxation of the nuclei.
© Copyright Hans J. Reich 2013
CHEM 430 – NMR Spectroscopy
3
NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
Measurement of Relaxation Time.
• A convenient method has been developed for measuring T1: inversion
recovery.
• A series of NMR spectra are measured in which the spins are inverted with a
180o pulse, followed by a variable waiting period τ.
• A second 90o pulse allows one to measure the amount of decay of the z-
component at this time τ:
CHEM 430 – NMR Spectroscopy
4
NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
Measurement of Relaxation Time.
© Copyright Hans J. Reich 2013
CHEM 430 – NMR Spectroscopy
5
NMR – Advanced
1-D Techniques
SPIN-LATTICE AND SPIN-SPIN RELAXATION
5-1
Measurement of Relaxation Time.
For each of the six carbons
in chlorobenzene, note
when the signal is nulled.
That is T1 !
CHEM 430 – NMR Spectroscopy
6
NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
• Observation:
• T1 relaxation times are longest for small and very large molecules.
• T1 relaxation times are lengthened by higher field instruments, in general.
• T1 relaxation minima is shifted towards smaller molecules on higher field
instruments.
© Copyright Hans J. Reich 2013
CHEM 430 – NMR Spectroscopy
7
NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
• Observation:
• tC or correlation time is a measure of the rotation and translational rates
of molecules in solution.
• By definition this rate will approach zero in highly viscous systems and
solids.
© Copyright Hans J. Reich 2013
CHEM 430 – NMR Spectroscopy
8
NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
Causes of Relaxation.
• T1 relaxation occurs because of the presence of natural magnetic fields in the
sample that fluctuate at the Larmor frequency--excess spin energy can flow
into the molecular surroundings or lattice
• The rate of spontaneous relaxation of nuclear spin orientations is practically
zero.
From: Hornack, J.P., http://www.cis.rit.edu/htbooks/nmr/
CHEM 430 – NMR Spectroscopy
9
NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
Causes of Relaxation.
• The major source of these magnetic fields is magnetic nuclei in motion or
the process of Dipole-Dipole Relaxation (DD)
• It involves the interaction of the resonating nuclear magnetic dipole with the
dipole of the nucleus in motion that causes the fluctuating field of the lattice.
From: Hornack, J.P., http://www.cis.rit.edu/htbooks/nmr/
CHEM 430 – NMR Spectroscopy
10
NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
• Correlation time (τc) for a molecule can be defined as roughly the interval
between two successive reorientations or positional changes of the molecule
(by vibration, rotation or translation)
• The correlation time for small molecules is of the order of 10-12 sec in
solution (longer in viscous solvents).
• For a proton at 300 MHz, νo ≈ 108, most molecules below molecular weight of
1000 are moving too rapidly for effective relaxation.
CHEM 430 – NMR Spectroscopy
11
NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
• The resulting relaxation time depends on
• The number of nearby nuclei - n
• Nuclear properties of both resonating and moving nuclei, g
• Distance between them, r -6
• Correlation time: tc
• Mathematically for 13C relaxed by protons:
• Mathematically for protons relaxed by protons:
CHEM 430 – NMR Spectroscopy
12
NMR – Advanced
1-D Techniques
•
13C
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
relaxation is faster when
• There are more attached protons
• The internuclear distance C—H is less
• When rotation in solution decreases
• Quaternary 13C has a long relaxation time because it lacks an attached proton
and because the distance rCH to other protons is large.
• The ratio of the 13C relaxation time of CH to CH2 to CH3 is 6: 3: 2 (1: ½ :⅓),
• Because the rate of molecular tumbling in solution slows as molecular size
increases, larger molecules relax more rapidly.
• Thus, cholesteryl chloride relaxes more rapidly than phenanthrene, which
relaxes more rapidly than benzene.
CHEM 430 – NMR Spectroscopy
13
NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
• The previous equations are an approximation to a more complete equation
and represents what is called the extreme narrowing limit for smaller
molecules.
• Because the frequency of motion of the moving nuclear magnet must match
the resonance frequency of the excited nuclear magnet, dipolar relaxation
becomes ineffective for both rapidly moving small molecules and slowly
moving large molecules.
• Many molecules of interest to biochemists fall into the latter category,
• Similarly rapid internal rotation of methyl groups in small molecules also
can reduce the effectiveness of dipole– dipole relaxation.
• The optimal correlation times tc for dipolar relaxation lie in the range of 10-7
to 10-11 s (inverse of the resonance frequency).
CHEM 430 – NMR Spectroscopy
14
NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
Structural Ramifications.
• Proton spin– lattice relaxation times depend on the distance between the
resonating nucleus and the nearest- neighbor protons.
• The closer the neighbors are, the faster is the relaxation - shorter T1.
• The two isomers (anomers) below may be distinguished by their proton
relaxation times:
H1 is eq
with more
distant
neighbors:
T1 of 4.1 s.
H1 is ax and close
to the 3 and 5 ax
protons, T1 of 2.0 s
CHEM 430 – NMR Spectroscopy
15
NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
• When dipolar relaxation is slow, other mechanisms of relaxation become
important. Fluctuating magnetic fields also can arise from:
1. Interruption of the motion of rapidly tumbling small molecules or
rapidly rotating groups within a molecule ( spin rotation relaxation),
2. Tumbling of molecules with anisotropic chemical shielding at high fields
3. Scalar coupling constants that fluctuate through chemical exchange or
through quadrupolar interactions
4. Tumbling of paramagnetic molecules (unpaired electrons have very large
magnetic dipoles)
5. Tumbling of quadrupolar nuclei
• In the absence of quadrupolar nuclei or paramagnetic species, these
alternative mechanisms often are unimportant.
CHEM 430 – NMR Spectroscopy
16
NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
Anisotropic Motion.
• When a molecule is rigid and rotates equally in any direction (isotropically),
all the carbon relaxation times (after adjustment for the number of attached
protons) should be nearly the same.
• The non-spherical shape of a molecule, however, frequently leads to
preferential rotation in solution around one or more axes (anisotropic
rotation).
Example: toluene rotates around the long axis so that less mass is in motion.
On average, these carbons (and their attached protons) move less in solution
than the ortho and meta carbons, because atoms on the axis of rotation
remain stationary during rotation.
CHEM 430 – NMR Spectroscopy
17
NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
Segmental Motion.
• When molecules are not rigid, the more rapidly moving pieces relax more
slowly because their tc is shorter.
• Example: In decane the methyl carbon relaxes most slowly, followed by the
ethyl carbon, and so on, to the fifth carbon in the middle of the chain.
• The structure gives the values of nT1 (n = # of attached protons), So these
values reflect the relative rates of motion of each carbon with the attached
protons normalized out:
CHEM 430 – NMR Spectroscopy
18
NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
Partially Relaxed Spectra.
• The inversion recovery experiment used to measure T1 also may be
exploited to simplify spectra: In the inversion recovery example the
spectrum for t = 40 s lacks the ipso carbon resonance
• Such partially relaxed spectra can be used not only to obtain partial spectra
in this fashion but also to eliminate specific peaks.
• When D2O is used as the solvent, the residual HOD peak is undesirable. An
inversion recovery experiment can reveal the value of for which the water
peak is nulled.
• Solvent suppression: Apply the 180° pulse selectively only at the resonance
position of water. Selection of t for nulling of this peak then produces a
spectrum that lacks the water peak but otherwise is quite normal for the
remaining resonances.
CHEM 430 – NMR Spectroscopy
19
NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
Partially Relaxed Spectra – Solvent Suppression
2mM Sucrose in H2O:D2O 9:1
HOD
CHEM 430 – NMR Spectroscopy
20
NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
Quadrupolar Relaxation.
• The dominant mode of spin– lattice relaxation for nuclei with spins greater than
½ results from the quadrupolar nature of such nuclei.
• These nuclei are considered to have an ellipsoidal rather than a spherical shape.
When I = 1, as for 14N or 2H, there are three stable orientations in the magnetic
field: parallel, orthogonal, and antiparallel:
• When these ellipsoidal nuclei tumble in solution within an unsymmetrical
electron cloud of the molecule, they produce a fluctuating electric field that can
bring about relaxation.
CHEM 430 – NMR Spectroscopy
21
NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
Quadrupolar Relaxation.
• The mechanism is different from dipole–dipole relaxation in two ways.
1. It does not require a second nucleus in motion; the quadrupolar nucleus
creates its own fluctuating field by moving in the unsymmetrical electron
cloud.
2. Because the mechanism is extremely effective when the quadrupole
moment of the nucleus is large, T1 can become very short ( < milliseconds).
• By the uncertainty principle the product of DE and t must remain constant:
(DE·t ~ h) ∴when the relaxation time is very short, the linewidth becomes very
large.
• Nuclei with large quadrupole moments often exhibit very large linewidths— for
example, about 20,000 Hz for the 35Cl resonance of CCl4.
CHEM 430 – NMR Spectroscopy
22
NMR – Advanced
1-D Techniques
5-1
SPIN-LATTICE AND SPIN-SPIN RELAXATION
Quadrupolar Relaxation.
• The common nuclides 17O and 14N have smaller quadrupolar moments and
exhibit sharper resonances, typically tens of hertz.
• The linewidth also depends on the symmetry of the molecule, which controls
how unsymmetrical the electron cloud is. Systems with p electrons are more
unsymmetrical and give broader lines
CHEM 430 – NMR Spectroscopy
23
NMR – Advanced
1-D Techniques
5-2
REACTIONS ON THE NMR TIME SCALE
• NMR is an excellent tool for following the kinetics of an irreversible reaction
traditionally through the disappearance or appearance of peaks over periods of
minutes to hours.
• The spectrum is recorded repeatedly at specific intervals, and rate constants are
calculated from changes in peak intensities.
• Thus, the procedure is a classical kinetic method, performed on the laboratory
time scale.
• The molecular changes take place on a time scale much longer than the pulse or
acquisition times of the NMR experiment.
CHEM 430 – NMR Spectroscopy
24
NMR – Advanced
1-D Techniques
5-2
REACTIONS ON THE NMR TIME SCALE
• More importantly, NMR has the unique capability for
study of the kinetics of reactions that occur at
equilibrium and that affect line shapes:
• EA in the range from 4.5 to 25 kcal·mol-1
• Rates in the range from 100 to 104 s-1.
• This NMR time scale refers to the rough equivalence of
the reaction rate in s-1 to the frequency spacing in Hz
• Remember the study of axial and equatorial protons in
cyclohexane-d11 as a function of temperature:
• When interchange of two such chemical
environments occurs faster than the frequency
differences between the two sites, the result is a
single peak
• When the interchange is slower than the frequency
differences, the NMR result is two distinct peaks
CHEM 430 – NMR Spectroscopy
25
NMR – Advanced
1-D Techniques
5-2
REACTIONS ON THE NMR TIME SCALE
Hindered Rotation.
• Normally, rotation around single bonds has a barrier below 5 kcal·mol-1 faster than the NMR time scale.
• Rotation around the double bond of alkenes, on the other hand, has a
barrier that is normally above 50 kcal·mol-1 and is slow on the NMR time
scale.
• There are numerous examples of intermediate bond orders, whose
rotation occurs within the NMR time scale. Hindered rotation about the
bond in amides such as N,N-dimethylformamide provides a classic
example of site exchange:
• RT - exchange is slow and two CH3 resonances are observed,
• > 100° C, exchange is fast and a single resonance is observed.
• Measured barrier is about 22 5 kcal·mol-1 .
CHEM 430 – NMR Spectroscopy
26
NMR – Advanced
1-D Techniques
5-2
REACTIONS ON THE NMR TIME SCALE
Hindered Rotation.
• Hindered rotation occurs on the NMR time scale for numerous other
systems with partial double bonds, including carbamates, thioamides,
enamines, nitrosamines, alkyl nitrites, diazoketones, aminoboranes, and
aromatic aldehydes.
• Formal double bonds can exhibit free rotation when alternative resonance
structures suggest partial single bonding.
• Calicene has a barrier to rotation about the central bond of 20 kcal·mol-1:
CHEM 430 – NMR Spectroscopy
27
NMR – Advanced
1-D Techniques
5-2
REACTIONS ON THE NMR TIME SCALE
Hindered Rotation.
• Steric congestion can raise the barrier about a single bond enough to bring
it into the NMR range.
• Rotation about the single bond in the biphenyl shown is raised to a
measurable 13 kcal·mol-1 by the presence of the ortho- substituents, which
also provide diastereotopic methylene protons as the dynamic probe:
CHEM 430 – NMR Spectroscopy
28
NMR – Advanced
1-D Techniques
5-2
REACTIONS ON THE NMR TIME SCALE
Hindered Rotation.
• Hindered rotation about an s–bond can sometimes be observed when at
least one of the carbons is quaternary.
• Example: At – 150 °C the tert-butyl group in tert-butylcyclopentane gives
two resonances in the ratio of 2: 1, since two of the methyl groups are
different from the third C :
CHEM 430 – NMR Spectroscopy
29
NMR – Advanced
1-D Techniques
5-2
REACTIONS ON THE NMR TIME SCALE
Hindered Rotation.
• Hindered rotation has frequently been observed in halogenated alkanes.
• The barrier probably arises from a combination of steric and electrostatic
interactions. 2,2,3,3-Tetrachlorobutane at - 40° C exhibits a 2: 1 doublet
below from anti and gauche rotamers that are rotating slowly on the NMR
time scale.:
• When both atoms about a s-bond possess lone electron pairs, the barrier
often (due to electrostatic interactions or repulsions) is often observable;
for example the S—S bond in dibenzyl disulfide 7 kcal·mol-1 .
CHEM 430 – NMR Spectroscopy
30
NMR – Advanced
1-D Techniques
5-2
REACTIONS ON THE NMR TIME SCALE
Ring Reversal.
• Axial-equatorial interconversion through ring reversal has been studied in
a wide variety of systems in addition to cyclohexane:
• 8-membered rings such as cyclooctane have been examined extensively;
The d15-derivative exhibits dynamic behavior below - 100° C, with an EA of
7.7 kcal·mol-1.
• Cyclooctatetraene undergoes a boat–boat ring reversal. side chain methyl
groups provide the diastereotopic probe to estimate the barrier of 14.7
kcal·mol-1.
CHEM 430 – NMR Spectroscopy
31
NMR – Advanced
1-D Techniques
5-2
REACTIONS ON THE NMR TIME SCALE
Atomic Inversion.
• Trisubstituted atoms with a lone pair, such as amines, may undergo the
process of pyramidal atomic inversion on the NMR time scale.
• The resonances of the two methyls in the aziridine become equivalent at
elevated temperatures through rapid N-inversion.
• The high barrier of 18 kcal·mol-1 is due to angle strain in the threemembered ring, which is higher in the transition state
• The effect is observed to a lesser extent in azetidines (9 kcal·mol-1) and in
strained bicyclic systems (10 kcal·mol-1).
CHEM 430 – NMR Spectroscopy
32
NMR – Advanced
1-D Techniques
5-2
REACTIONS ON THE NMR TIME SCALE
Atomic Inversion.
• The inversion barrier may be raised when nitrogen is attached to highly
electronegative elements.
• This substitution increases the s character of the ground- state lone pair.
Since the transition- state lone pair must remain p- hybridized, the barrier
is higher, as in N- chloropyrrolidine:
• When neither ring strain nor electronegative substituents are present,
barriers are lower, as in N- methylazacycloheptane 7 kcal·mol-1
CHEM 430 – NMR Spectroscopy
33
NMR – Advanced
1-D Techniques
5-2
REACTIONS ON THE NMR TIME SCALE
Atomic Inversion.
• Inversion barriers for elements in lower rows of the periodic table
generally are above the NMR range: chiral phosphines and sulfoxides are
isolable.
• Barriers must be brought into the observable NMR range by substitution
with electropositive elements, as in diphosphine CH3(C6H5)P—P(C6H5)CH3,
with a barrier of 26 kcal·mol- 1
• The barrier in phosphole is lowered because the transition state is
aromatic. Compare its barrier of 16 kcal·mol- 1 to 36 kcal·mol- 1 in a
saturated analogue:
CHEM 430 – NMR Spectroscopy
34
NMR – Advanced
1-D Techniques
5-2
REACTIONS ON THE NMR TIME SCALE
Valence Tautomerizations and Bond Shifts.
• The barriers to many valence tautomerizations fall into the NMR range.
• A classic example is the Cope rearrangement of 3,4- homotropilidine, at
low temperatures, the spectrum has the features expected for the five
functionally distinct types of protons (disregarding diastereotopic differences).
• At higher temperatures, the rearrangement becomes fast on the NMR time
scale, and only three types of resonances are observed (barrier of 14
kcal·mol- 1 for the 1,3,5,7- tetramethyl derivative).
CHEM 430 – NMR Spectroscopy
35
NMR – Advanced
1-D Techniques
5-2
REACTIONS ON THE NMR TIME SCALE
Valence Tautomerizations and Bond Shifts.
• When a third bridge is added, as in barbaralone steric requirements of the
rearrangement are improved, and the barrier is lowered to 9.6 kcal·mol- 1 .
• When the third bridge is an ethylenic group, the molecule is bullvalene
where all three bridges are identical, and a sequence of Cope
rearrangements renders all protons (or carbons) equivalent.
• Indeed, the complex spectrum at room temperature becomes a singlet
above 180 oC.
• Molecules that undergo rapid valence tautomerizations often are said to be
fluxional.
CHEM 430 – NMR Spectroscopy
36
NMR – Advanced
1-D Techniques
5-2
REACTIONS ON THE NMR TIME SCALE
Valence Tautomerizations and Bond Shifts.
• Rearrangements of carbocations also may be studied by NMR methods.
• The norbornyl cation may undergo 3,2-and 6,2-hydride shifts, as well as
Wagner–Meerwein rearrangements.
• The sum of these processes renders all protons equivalent, so that the
complex spectrum below -80 oC becomes a singlet at room temperature.
• The slowed process appears to be the 3,2- hydride shift, whose barrier was
measured to be 11 kcal·mol- 1
CHEM 430 – NMR Spectroscopy
37
NMR – Advanced
1-D Techniques
5-2
REACTIONS ON THE NMR TIME SCALE
Valence Tautomerizations and Bond Shifts.
• Fluxional organometallic species have also been observed with NMR
• Tetra-methylalleneiron tetracarbonyl exhibits three distinct methyl
resonances in the ratio 1: 1: 2 at - 60° as depicted:
• Above room temperature, however, the spectrum becomes a singlet as the
Fe(CO)4 unit circulates about the allenic structure by moving orthogonally
from one alkenic unit to the other.
CHEM 430 – NMR Spectroscopy
38
NMR – Advanced
1-D Techniques
5-2
REACTIONS ON THE NMR TIME SCALE
Valence Tautomerizations and Bond Shifts.
• In cyclooctatetreneiron tricarbonyl, the spectrum below –150 °C indicates
four protons on carbons bound to iron and four on carbons not bound to
iron, consistent with the structure shown.
• Above –100 °C all the protons converge to a singlet as the iron atom moves
around the ring as shown.
• A bond shift occurs with each 45° movement of the iron atom. Eight such
operations result in complete averaging of the ring protons or carbons.
CHEM 430 – NMR Spectroscopy
39
NMR – Advanced
1-D Techniques
5-2
REACTIONS ON THE NMR TIME SCALE
Quantification.
• For the simple case of two equally populated sites that
do not exhibit coupling (ex: cyclohexane) the rate
constant (kc) at the point of maximum peak
broadening (the coalescence temperature Tc, d11
approximately - 60° C) is
kc = pDn/ √2
• Dn is the distance in Hz between the two peaks at slow
exchange.
• The free energy of activation then may be calculated as
DGc‡ = 2.3RTc [10.32 + log(Tc / kc )]
• This result is extremely accurate and easy to obtain,
but the equation is limited in its application.
CHEM 430 – NMR Spectroscopy
40
NMR – Advanced
1-D Techniques
5-2
REACTIONS ON THE NMR TIME SCALE
Quantification.
• For the two- site exchange between coupled nuclei the rate constant at Tc
kc = p(Dn2 + 6J2)½ / √2
• To include unequal populations, more complex coupling patterns, and
more than two exchange sites, it is necessary to use computer programs
such as DNMR3, which can simulate the entire line shape at several
temperatures.
• Such a procedure generates Arrhenius plots from which enthalpic and
entropic activation parameters may be obtained.
• The procedure is more elegant and more comprehensive, but it is more
susceptible to systematic errors involving inherent linewidths and peak
spacings than is the coalescence temperature method.
CHEM 430 – NMR Spectroscopy
41
NMR – Advanced
1-D Techniques
5-2
REACTIONS ON THE NMR TIME SCALE
Quantification.
• The proportionality between kc and Dn means that the rate constant is
dependent on the field strength
• Thus, a change in field from 300 to 600 MHz alters the rate constant at Tc.
The practical result is that changes.
• Since the slow exchange peaks are farther apart at 600 MHz, a higher
temperature is required to achieve coalescence than at 300 MHz.
• At a given field strength, two nuclides such as and have different values of
for Dn analogous functionalities and achieve coalescence at different
temperatures.
• Since Dn is usually larger for than 1H than for 13C the coalescence temperature often is much higher for 13C.
CHEM 430 – NMR Spectroscopy
42
NMR – Advanced
1-D Techniques
5-3
MULTIPLE RESONANCE
• Special effects may be routinely and elegantly created by using sources of
radiofrequency energy in addition to the observation frequency , n1 = gB1
• The technique is called multiple irradiation or multiple resonance and
requires the presence of a second transmitter coil in the sample probe to
provide the new irradiating frequency n2 = gB2
• When the second frequency is applied, the experiment, which is widely
available on modern spectrometers, is termed double resonance or
double irradiation.
• We already have seen several examples of double irradiation experiments,
including the removal of proton couplings from 13C, the elimination of
solvent peaks by peak suppression, the sharpening of NH resonances by
irradiation of 14N.
CHEM 430 – NMR Spectroscopy
43
NMR – Advanced
1-D Techniques
5-3
MULTIPLE RESONANCE
Spin Decoupling.
• One of the oldest and most generally applicable double-resonance
experiments is the irradiation of one proton resonance (HX) and
observation of the effects on the AX coupling (JAX) present in another
proton resonance (HA).
• The traditional and intuitive explanation for the resulting spectral
simplification, known as spin decoupling, is that the irradiation shuttles
the X protons between the spin states so rapidly that the A protons no
longer have a distinguishable independent existence.
• As a result, the A resonance collapses to a singlet. This explanation,
however, is inadequate in that it fails to account for phenomena at weak
decoupling fields (spin tickling) and even some phenomena at very strong
decoupling fields.
CHEM 430 – NMR Spectroscopy
44
NMR – Advanced
1-D Techniques
5-3
MULTIPLE RESONANCE
Spin Decoupling.
• The actual experiment involves getting the coupled nuclei to precess about
orthogonal axes.
• The magnitude of the coupling interaction between two spins is expressed
by the scalar, or dot, product between their magnetic moments and is
proportional to the expression:
Jm1 · m2 = Jm1m2cosf
• The quantity f is the angle between the vectors (the axes of precession of
the nuclei).
• So long as both sets of nuclei precess around the same (z) axis, f is zero,
cos 0o is 1, and full coupling is observed.
CHEM 430 – NMR Spectroscopy
45
NMR – Advanced
1-D Techniques
MULTIPLE RESONANCE
5-3
Spin Decoupling.
• The geometrical relationship between the spins may be altered by
subjecting one of them to a B2 field.
• Imagine observing
frequency B2.
13C
nuclei as they precess around the z axis at the
• When the attached protons are subjected to a strong B2 field along the x
axis, they will precess around that axis.
• The angle f between the 1H and 13C nuclear vectors is 90° as they
respectively precess around the z and x axes.
• As a result, their spin– spin interaction goes to zero because the dot
product is zero . The nuclei are then said to be decoupled.
CHEM 430 – NMR Spectroscopy
46
NMR – Advanced
1-D Techniques
MULTIPLE RESONANCE
5-3
Spin Decoupling.
• Spin decoupling has been useful
in identifying coupled pairs of
nuclei.
• Consider ethyl trans-crotonate,
the alkenic protons split each
other, and both are split by the
allylic methyl group to form an
ABX3 spin system.
• Irradiation
at the methyl
resonance frequency produces
the upper spectrum in the inset
for the alkenic protons, which
have become a simple AB
quartet.
CHEM 430 – NMR Spectroscopy
47
NMR – Advanced
1-D Techniques
5-3
MULTIPLE RESONANCE
Spin Decoupling.
• A more complex example is illustrated using the bicyclic sugar mannosan
triacetate, which has a nearly first-order spectrum with numerous coupling
partners.
• Irradiation of H5 @ d 4.62 produces simplification of the resonances of its
vicinal partners H4, H6/1 H6/2 as well as its long- range zigzag partner H3:
CHEM 430 – NMR Spectroscopy
48
NMR – Advanced
1-D Techniques
5-3
MULTIPLE RESONANCE
Difference Decoupling.
• In complex molecules, the difference between coupled and decoupled
spectra can be used as a probe for nuclear relationships
• Features that are not affected by decoupling are subtracted out and do not
appear.
• The procedure provides coupling relationships when spectral overlap is a
serious problem.
• This and other simple spin-decoupling experiments have been entirely
superseded by two- dimensional experiments in recent years
CHEM 430 – NMR Spectroscopy
49
NMR – Advanced
1-D Techniques
5-3
MULTIPLE RESONANCE
Difference Decoupling.
• Consider the 1H spectrum of 1- dehydrotestosterone:
b) irradiation of the
6 resonance shows
little change as the
result of double
irradiation. But if
the original
spectrum (a) is
subtracted from
(b), the remaining
resonances must be
from the effect of
the 7 protons (c).
CHEM 430 – NMR Spectroscopy
50
NMR – Advanced
1-D Techniques
5-3
MULTIPLE RESONANCE
Classes of Multiple Resonance Experiments.
• Homonuclear double-resonance experiments: both the irradiated and the
observed nuclei are identical – represented by the notation 1H{1H}.
(irradiated nucleus is denoted by braces)
• Heteronuclear double-resonance experiements: the observed and irradiated
nuclei are different – denoted by the notation 13C{1H} (as in protondecoupled 13C spectra) or 13C{1H}{31P} for a triple-resonance experiment.
• Double-resonance experiments also may be classified according to the
intensity or bandwidth of the irradiating frequency.
• If irradiation is intended to cover only a portion of the resonance
frequencies, the technique is known as selective irradiation or selective decoupling.
CHEM 430 – NMR Spectroscopy
51
NMR – Advanced
1-D Techniques
5-3
MULTIPLE RESONANCE
Classes of Multiple Resonance Experiments.
• Non-irradiated resonances can exhibit a small movement in frequency, called
the Bloch–Siegert shift, which is related to the intensity of the B2 field and the
distance between the observed and irradiated frequencies.
• Closer examination of the mannosan triacetate experiment reveals several
such shifts, found by comparing the relative positions of the resonances in
the upper and lower spectra:
CHEM 430 – NMR Spectroscopy
52
NMR – Advanced
1-D Techniques
5-3
MULTIPLE RESONANCE
Classes of Multiple Resonance Experiments.
• When all frequencies of a specific nuclide are irradiated, the experiment is
termed nonselective irradiation or broadband decoupling.
• The invention of this technique was instrumental in the development of 13C
NMR spectroscopy as a routine tool. To cover all the 1H frequencies, B2 was
modulated with white noise (previously), so the technique often was called
noise decoupling.
CHEM 430 – NMR Spectroscopy
53
NMR – Advanced
1-D Techniques
5-3
MULTIPLE RESONANCE
Classes of Multiple Resonance Experiments.
• There are two significant problems with this method.
1. Application of rf energy at the decoupling frequency generates heat - As
B0 fields increased from 60 to 900 MHz, higher decoupling intensities
were required generating heat that was unacceptable for biological
samples and for many delicate organic or inorganic samples.
2. Second, with higher field strengths, it became increasingly more difficult
for B2 to cover the entire range of 1H frequencies, which had been about
600 Hz at 60 MHz, but became 5000 Hz at 500 MHz.
• To overcome these problems of heteronuclear decoupling, modern methods
replaced continuous irradiation with a series of pulses that eliminate the
effects of coupling.
CHEM 430 – NMR Spectroscopy
54
NMR – Advanced
1-D Techniques
5-3
MULTIPLE RESONANCE
Classes of Multiple Resonance Experiments.
• In this experiment a 90° B1 pulse applied to the observed 13C nuclei along the
x direction moves magnetization from carbon coupled to either spin-up or
spin- down protons into the xy plane along the y axis:
CHEM 430 – NMR Spectroscopy
55
NMR – Advanced
1-D Techniques
5-3
MULTIPLE RESONANCE
Classes of Multiple Resonance Experiments.
• The reference frequency is considered to coincide with the y axis and be
midway between the frequencies of the carbons associated with the spin-up
Ha and spin-down Hb protons.
CHEM 430 – NMR Spectroscopy
56
NMR – Advanced
1-D Techniques
5-3
MULTIPLE RESONANCE
Classes of Multiple Resonance Experiments.
• The two carbon vectors then diverge in the xy plane after the 90° pulse, one
becoming faster and the other slower than the carrier frequency
CHEM 430 – NMR Spectroscopy
57
NMR – Advanced
1-D Techniques
5-3
MULTIPLE RESONANCE
Classes of Multiple Resonance Experiments.
• Then a 180° proton pulse (B2 of the decoupling experiment) switches the
locations of the vectors. The slower-moving vector that was dropping behind
is replaced by the faster- moving vector and vice-versa, so that both carbon
vectors start to move back toward the y axis:
CHEM 430 – NMR Spectroscopy
58
NMR – Advanced
1-D Techniques
5-3
MULTIPLE RESONANCE
Classes of Multiple Resonance Experiments.
• After an equal second period , the two vectors coincide on the y axis, only one
frequency or peak occurs, and coupling to the protons disappears The
process is repeated during acquisition at a rate (in hertz) that is faster than
the coupling constant, so that the effects of coupling are removed.
CHEM 430 – NMR Spectroscopy
59
NMR – Advanced
1-D Techniques
5-3
MULTIPLE RESONANCE
Classes of Multiple Resonance Experiments.
• In this way, decoupling can be achieved with short pulses during acquisition
rather than with a continuous, high-intensity field during the entire
experiment.
• In practice, the method is limited because the 180° pulse must be very
accurate and because the B2 field is inhomogeneous.
• Refinements of this experiment have been achieved by replacing the 180°
pulse with several pulses (composite pulses) and by cycling their order (
phase cycling) to cancel out the inaccuracies
• One of these methods is WALTZ-16, which achieves full decoupling across a
much wider range than the original continuous method and with a fraction of
the power – used by our Bruker 400
CHEM 430 – NMR Spectroscopy
60
NMR – Advanced
1-D Techniques
5-4
THE NUCLEAR OVERHAUSER EFFECT
Introduction.
• Dipole–dipole relaxation occurs when two nuclei are located close together
and are moving at an appropriate relative rate
• Irradiation of one of these nuclei with a B2 field alters the Boltzmann
population distribution of the other nucleus and therefore perturbs the
intensity of its resonance.
• No J coupling need be present between the nuclei. The original phenomenon
was discovered by Overhauser, but between nuclei and unpaired electrons.
• The Overhauser effect when both spins are of nuclei was observed first by
Anet and Bourne and is of more interest to the chemist. It has great
structural utility, because the dipole–dipole mechanism for relaxation
depends on the distance between the two spins
CHEM 430 – NMR Spectroscopy
61
NMR – Advanced
1-D Techniques
THE NUCLEAR OVERHAUSER EFFECT
5-4
Origin.
• Consider a molecule that contains a
13C atom and a nearby (though not
necessarily spin-coupled) 1H atom.
C
H
N4
C
H
• The combinations of spin states for N3
this two-spin system are shown:
• Energy is the vertical axis.
• By Boltzmann the N1 state has a
very large population, but we
observe NMR signals from the small
populations in N2, N3 and N4.
CHEM 430 – NMR Spectroscopy
C
H
N2
C
H
N1
62
NMR – Advanced
1-D Techniques
5-4
THE NUCLEAR OVERHAUSER EFFECT
Origin.
• Red arrows indicate the resonance n
of the 13C nuclei (resulting in the 13C
signal on the spectrum)
C
H
N4
C
H
nC
• Blue arrows indicate the resonance N3
nH of the 1H nuclei (resulting in the
1H signal on the spectrum)
• Remember that the resonance nH is
four times that of nC
nH
C
nH
H
N2
C
nC
H
N1
CHEM 430 – NMR Spectroscopy
63
NMR – Advanced
1-D Techniques
5-4
THE NUCLEAR OVERHAUSER EFFECT
Origin.
• By the Boltzman distribution we
know that at equilibrium the
population of carbon and proton is
slightly larger in the lower energy
state (+½)
C
H
N4
C
H
nC
N3
nH
• We denote the excess population as
DC for carbon and DH for proton, so:
State Population
N4
- DH - DC
N3
- DH + DC
N2
+DH - DC
N1
+DH + DC
C
nH
H
N2
C
nC
H
N1
CHEM 430 – NMR Spectroscopy
64
NMR – Advanced
1-D Techniques
5-4
THE NUCLEAR OVERHAUSER EFFECT
Origin.
• For the carbon signals the energy
difference between the N1 to N2 and
N3 to N4 states is 2DC as shown in
the table below.
• We simply subtract one population
from the other to get the difference
between states:
C
H
N4
C
H
nC
N3
nH
C
State
Population
Difference
N4
- DH - DC
N3
- DH + DC
N2
+DH - DC
N1
+DH + DC
} 2D
} 2D
nH
C
H
N2
C
nC
H
N1
C
CHEM 430 – NMR Spectroscopy
65
NMR – Advanced
1-D Techniques
5-4
THE NUCLEAR OVERHAUSER EFFECT
Origin.
• The 13C signal we observe results
from N1 N2 and N3 N4
transitions where the population
difference is 2DC in both cases.
C
H
N4
C
H
nC
N3
nH
C
State
Population
Difference
N4
- DH - DC
N3
- DH + DC
N2
+DH - DC
N1
+DH + DC
} 2D
} 2D
nH
C
H
N2
C
nC
H
N1
C
CHEM 430 – NMR Spectroscopy
66
NMR – Advanced
1-D Techniques
5-4
THE NUCLEAR OVERHAUSER EFFECT
Origin.
• Now let’s saturate the 1H states by
double resonance decoupling.
• DH now becomes zero disturbing the
Boltzmann controlled equillibrium
C
H
N4
C
H
nC
N3
nH
• DH goes to zero
C
State
Population
Difference
N4
- DC
N3
+ DC
N2
- DC
N1
+ DC
} 2D
} 2D
nH
C
H
N2
C
nC
H
N1
C
CHEM 430 – NMR Spectroscopy
67
NMR – Advanced
1-D Techniques
5-4
THE NUCLEAR OVERHAUSER EFFECT
Origin.
• The populations of the N1 and N4
states are now polarized.
C
H
N4
C
H
nC
N3
nH
• In an attempt to restore equilibrium
a number of N4 carbon nuclei relax
to the N1 state by Dr
State
C
Population
Difference
N4
- DC
N3
+ DC
} 2D + D
N2
- DC
N1
+ DC
C
} 2D + D
C
nH
r
H
N2
C
nC
H
N1
r
CHEM 430 – NMR Spectroscopy
68
NMR – Advanced
1-D Techniques
5-4
THE NUCLEAR OVERHAUSER EFFECT
Origin.
• This leakage caused by saturation
and polarization of the N4 state by
Dr will increase the population of N1
and therefore increase the signal
detected
C
H
N4
C
H
nC
N3
nH
• This is the Nuclear Overhauser
Effect!
C
State
Population
Difference
N4
- DC
N3
+ DC
} 2D + D
N2
- DC
N1
+ DC
C
} 2D + D
C
nH
r
H
N2
C
nC
H
N1
r
CHEM 430 – NMR Spectroscopy
69
NMR – Advanced
1-D Techniques
5-4
THE NUCLEAR OVERHAUSER EFFECT
Quantification.
• The NOE is an example of polarization transfer – polarization of one set of
nuclear spin states results in the polarization of another
• For small molecules the maximum effect h is limited by the following
equation:
• The increase is always less than maximum as other non-polar relaxation
processes compete and the observed nucleus can also relax through other
non-irradiated nuclei
• For homonuclear NOE girr = gobs so the maximum effect is a ½ or 50%
enhancement of signal
CHEM 430 – NMR Spectroscopy
70
NMR – Advanced
1-D Techniques
5-4
THE NUCLEAR OVERHAUSER EFFECT
Quantification.
• For the heteronuclear 13Cobs/1Hirr system the g for 1H to 13C is ~ 4:1
• NOE enhancement is the principle reason why 13CH3- and –13CH2- signals are
enhanced in 1H-decoupled spectra relative to quaternary carbons
• The maximum enhancement for 13Cobs/1Hirr system is ~200%
• In general:
1. NOE experiments work best when girr ⩾ gobs
2. The signal enhancement is usually positive, however some nuclei (15N)
have negative g – you will see a negative peak!
CHEM 430 – NMR Spectroscopy
71
NMR – Advanced
1-D Techniques
5-4
THE NUCLEAR OVERHAUSER EFFECT
Difference NOE.
CHEM 430 – NMR Spectroscopy
72
NMR – Advanced
1-D Techniques
5-4
THE NUCLEAR OVERHAUSER EFFECT
Difference NOE.
CHEM 430 – NMR Spectroscopy
73
NMR – Advanced
1-D Techniques
SPECTRAL EDITING
5-5
Spin-Echo Experiment
• How can you know what T2 is?
• We cannot measure it directly like T1
• Pulse sequence is used called spin-echo sequence
90y
180y (or x)
tD
CHEM 430 – NMR Spectroscopy
tD
74
NMR – Advanced
1-D Techniques
SPECTRAL EDITING
5-5
Spin-Echo Experiment
• What it does:
z
y
x
y
tD
x
x
y
y
dephasing
y
tD
x
x
180y (or x)
refocusing
CHEM 430 – NMR Spectroscopy
75
NMR – Advanced
1-D Techniques
SPECTRAL EDITING
5-5
Spin-Echo Experiment
• What it does:
z
y
x
y
tD
x
x
y
y
dephasing
y
tD
x
x
180y (or x)
refocusing
CHEM 430 – NMR Spectroscopy
76
NMR – Advanced
1-D Techniques
5-5
SPECTRAL EDITING
Spin-Echo Experiment
• If we acquire the FID right after the spin-echo sequence, the
intensity of the signal after FT will only be affected by T2 relaxation
and not by dephasing due to B0 imperfections.
• Upon repetition for different tD values, we plot the intensity versus
intensity
2 * tD and get a graph similar to the one we got for inversion
recovery, but in this case the decay rate will be equal to T2.
time
I(t) = I * ( 1 - 2 * e - t / T2 )
CHEM 430 – NMR Spectroscopy
77
NMR – Advanced
1-D Techniques
5-5
SPECTRAL EDITING
The Attached Proton Test
• Very similar to the Spin-Echo Sequence, except we will use JCHn to adjust the
time between pulses:
• For the methine resonance – note how after the 90o pulse the C vector
splits into a doublet (one vector +, the other -). Another 180o pulse in the
y-coordinate has them refocus on the – y-axis.
CHEM 430 – NMR Spectroscopy
78
NMR – Advanced
1-D Techniques
5-5
SPECTRAL EDITING
The Attached Proton Test
• If the same experiment were carried out for a CH2 system:
• For the CH2 resonance, the middle peak will remain on the y-axis (middle
of triplet), here we wait 1/2J for a second period and repulse the sample to
bring the – vector we saw before with –CH- back to the + y-axis.
CHEM 430 – NMR Spectroscopy
79
NMR – Advanced
1-D Techniques
SPECTRAL EDITING
5-5
The Attached Proton Test
• Similar to the methine system,
the pulse sequence will give a
negative peak for CH3 and a
positive peak will result from
quaternary carbons with no
splitting by H.
• Proton irradiation (selective
pulses
like
WALTZ-16)
carefully
decouples
the
spectrum.
• At
right is the APT for
cholesteryl acetate (C, CH2
positive, CH and CH3 negative)
CHEM 430 – NMR Spectroscopy
80
NMR – Advanced
1-D Techniques
SPECTRAL EDITING
5-5
The Attached Proton Test
• Similar to the methine system,
the pulse sequence will give a
negative peak for CH3 and a
positive peak will result from
quaternary carbons with no
splitting by H.
• Proton irradiation (selective
pulses
like
WALTZ-16)
carefully
decouples
the
spectrum.
• At
right is the APT for
cholesteryl acetate (C, CH2
positive, CH and CH3 negative)
CHEM 430 – NMR Spectroscopy
81
NMR – Advanced
1-D Techniques
SPECTRAL EDITING
5-5
DEPT – Distortionless Enhancement by Polarization Transfer
• A sequence that takes advantage of the surplus 1H population to see 13C
signals and it can edit the signals in order to obtain response from CH,
CH2 and CH3 according to the settings of the sequence:
90x
180x
180x
fy
13C:
90x
tD
tD
tD
{1H}
1H:
CHEM 430 – NMR Spectroscopy
82
NMR – Advanced
1-D Techniques
5-5
SPECTRAL EDITING
DEPT – Distortionless Enhancement by Polarization Transfer
• As we just pointed out, the relaxation times for 13C are strongly a factor
of T2 processes – where spin is transferred and the resulting signal shifts
out of phase with what is being detected
• If different pulse widths are applied in a 13C determination, the resulting
phase shift (and signal intensity) will be different depending on how
many 1H atoms the 13C can transfer its spin to
• In a typical DEPT experiment, a 45, 90 and 135 pulse are applied
successively to the sample and the results compared to the original
carbon spectrum
• Unfortunately, it relies on the creation and manipulation of multiple
quantum magnetization (the 13C p/2 pulse) which we cannot see or
represent with vectors.
CHEM 430 – NMR Spectroscopy
83
NMR – Advanced
1-D Techniques
SPECTRAL EDITING
5-5
DEPT – Distortionless Enhancement by Polarization Transfer
• If we plot the responses for different carbons versus the tip angle f of
the 1H pulse, we get:
45
90
135
CH
CH2
CH3
CHEM 430 – NMR Spectroscopy
84
NMR – Advanced
1-D Techniques
5-5
SPECTRAL EDITING
DEPT – Distortionless Enhancement by Polarization Transfer
• In summary:
• At 45o we observe all
positive 13C resonances
except 4o
• At 90o we observe no
13C resonances for CH ,
2
o
CH3 or 4
• At 135o we observe
positive resonances for
CH and CH3, negative
resonances for CH2 and
none for 4o
CHEM 430 – NMR Spectroscopy
85
NMR – Advanced
1-D Techniques
SPECTRAL EDITING
5-5
DEPT – Distortionless Enhancement by Polarization Transfer
• Most useful to run is the DEPT-135 and if needed a DEPT-90:
DEPT Pulse
Sequence
methyl
methylene
methine
quaternary
DEPT-45
Positive peak
Positive peak
Positive peak
Not observed
DEPT-90
No obs. peak
No obs. peak
Positive peak
Not observed
DEPT-135
Positive peak
Negative Peak
Positive Peak
Not observed
CHEM 430 – NMR Spectroscopy
86
NMR – Advanced
1-D Techniques
SPECTRAL EDITING
5-5
DEPT – Distortionless Enhancement by Polarization Transfer
• Most useful to run is the DEPT-135 and if needed a DEPT-90:
DEPT Pulse
Sequence
methyl
methylene
methine
quaternary
DEPT-45
Positive peak
Positive peak
Positive peak
Not observed
DEPT-90
No obs. peak
No obs. peak
Positive peak
Not observed
DEPT-135
Positive peak
Negative Peak
Positive Peak
Not observed
CHEM 430 – NMR Spectroscopy
87
SPECTRAL EDITING
DEPT – Distortionless Enhancement by Polarization Transfer
• Example:
CHEM 430 – NMR Spectroscopy
88
NMR – Advanced
1-D Techniques
5-5
SPECTRAL EDITING
DEPT – Distortionless Enhancement by Polarization Transfer
• Example:
DEPT 135
DEPT 90
DEPT 45
CHEM 430 – NMR Spectroscopy
89