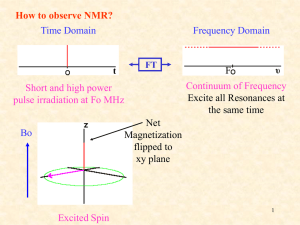
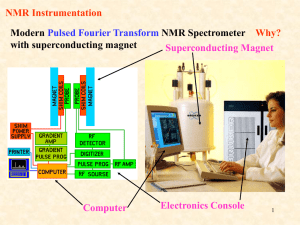
NMR to study membrane proteins
advertisement

Investigations of Membrane Polypeptides by Solid-state NMR Spectroscopy: Structure, Dynamics, Aggregation and Topology of Supramolecular Complexes Burkhard Bechinger Université Louis Pasteur, CNRS - UMR Chimie-physique moléculaire et spectroscopie Strasbourg, France NMR to study membrane proteins Solution NMR Requires fast and isotropic motional averaging < 120 kDa (TROSY) NMR to study membrane proteins Solution NMR Solid-state NMR Requires fast and isotropic motional averaging < 120 kDa (TROSY) frozen, dry liquid crystalline membranes Extended liquid crystalline bilayers are too big no physical size limitation Structure, orientation and dynamics Solid-state NMR provides information on … • • • • chemical environment distances dihedral angles orientations in space Structure, dynamics and topology Oriented membranes Bo Chemical synthesis of peptides allows labelling at single sites resin Deblock Activate aa resin Wash to reactor Couple resin Wash resin Oriented Samples: Structure and Topology 15N chemical shift alignment of the peptide bond Solution and solid-state NMR on the same scale The 2H quadrupolar splitting 2H 3-alanine 90° Bo DnQ ~ 3 cos2Q-1 60° 53° Q 30° Similar principles apply for many NMR interactions 0° Detailed helix alignment from combined 15N and 2H measurements 2 angles 2 measurables 175 ppm 20 125 tilt angle 150 100 75 50 25 25 50 75 100 125 rotational pitch angle 150 175 10 0 -10 kHz Unique solution from Energy Minimization hydrophobic hydrophilic + + + + + Tilt 95o, pitch 173o + KL14 Model Peptide in Oriented Phosphatidylcholine Bilayers Lipid POPC DMPC PC20:1 DOPC 2H (kHz) 15N (ppm) 6.0 7.6 8.3 10.8 74 73 73 74 Difference 2o Dynamics: Rotational Diffusion and Aggregation Liquid crystalline membranes Motion around the membrane normal || 11 33 22 Rotational averaging: Effect on 15N powder pattern line shape 2 2 1 1 Static 3 3 Rotation around 33 (helix long axis) Rotation around 22 33 11 22 || 33 22 2 2 250 200 150 100 11 50 ppm Powder pattern provide orientational information 2H solid-state NMR 2H -alanyl 3 Bo Bo Freezing Rotational Diffusion IP helix TM helix 313 K 303 K 293 K 283 K Loss of intensity during transition 263 K 50 0 kHz Equilibrium: Mono- / oligomer 2H-NMR Bo Bo 20 10 0 -10 -20 kHz 20 10 0 -10 -20 kHz 2H NMR of ‘‘real‘‘ samples e.g. viral channel peptides 20 0 Influenza M2 -20 kHz 20 0 Vpu kHz 2H spectral line shape and mosaic spread 10o Tilt angle: 40o 50o 700 Mosaic Spread 0.5 1 3 5 10 15 Model amphipathic helix 20 10 0 -10 -20 kHz Q = 45.3o or 65.5o Mosaic spread = 1o Example: Controlling Topology Oriented 15N solid-state NMR: LAH4 pH-dependent molecular switch pH < 5 pH 6.1 pH > 7 Example: Domain of ICP47 • Herpes simplex virus • 87 residues early gene product (domain 2-34 active) • Inhibits transport by TAP of antigenic peptides to surface and thus presentation by MHC I lack of immunogenic response • Solution NMR: Helix (5-14)-loop-helix (22-31) in SDS micelles c/o Robert Tampé - Frankfurt 15N solid-state NMR of ICP47(2-34) in oriented POPC 6 D13 E Helix1 T10 E6 9 D 13 N14 helix 1 M7 L5 L12 11 L F 8 A tilt 84o F A8 11 ‚Modelling‘ A K31 30 23 D24 N A,N27 Helix2 A,N27 23 A 24 D tilt 75o N14 L5 M7 12 Loop D T10 D9 helix 2 R26 R26 E28 E28 22 Y 22 Y I29 V25 K31 N30 V25 I29 2H solid-state NMR of ICP47(2-34) in oriented POPC Mosaic spread 10-15o Model for membrane-bound ICP47 Acknowledgements • • • • Christopher Aisenbrey Christina Sizun Bas Vogt Jesus Raya • Gérard Nullans, ULP-INSERM Neurochimie • Robert Tampé, Universität Frankfurt € ARC, ANRS, Vaincre la Mucoviscidose Region Alsace CNRS, Ministère, ACI Jeune Equipe Methods to orient lipid bilayers G la u b it z e t a l . Combine MAS and oriented samples N ew MAOSS at 10 kHz 31P NMR of oriented bilayers 10 kHz 565 Hz simulated Powder Parallel Perpendicular Experiment MAS side band analysis provides orientational information MAOSS of hydrophobic model peptide in phospholipid bilayer 15N PD PD 31P NMR NMR PD PD PD PD 250 200 150 100 50 ppm =10o 3.7o mosaic =25o 20 % powder 250 200 150 100 50 ppm 30 20 10 0 -20 ppm Summary • MAOSS with new sample set up low or fast spinning • spinning side band analysis tilt, mosaic spread and powder pattern contributions Model for membrane-bound ICP47 22 14 5 31