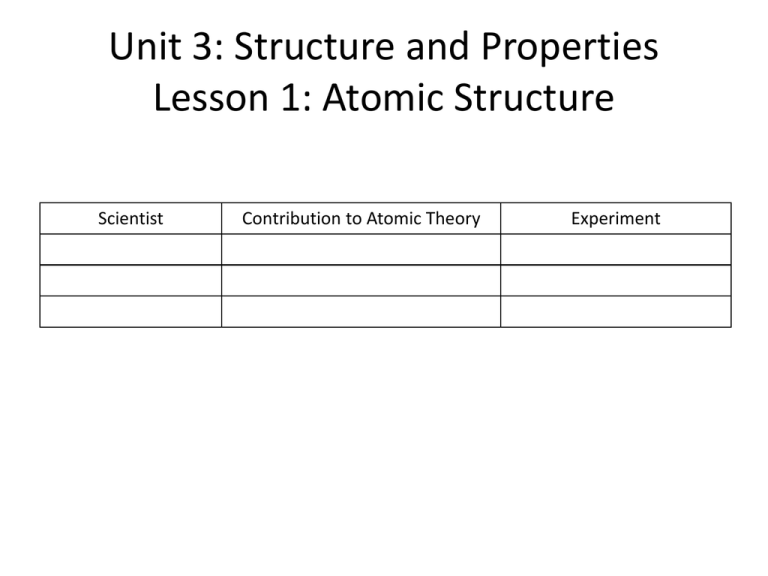
Unit 3: Structure and Properties
Lesson 1: Atomic Structure
Scientist
Contribution to Atomic Theory
Experiment
Atomic Structure
Chemistry 12: Chapter 4
Atomic Structure
You should be able to:
•Discuss the development of the atom from earliest atomic theory to modern
day theory of the atom
•Explain how experimental observations and inferences by Rutherford and
Bohr contributed to the development of the planetary model of the hydrogen
atom.
•Use appropriate terminology related to atomic structure including orbital,
emission spectrum, energy level, photon etc
•Describe the electron configurations of elements, using the concept of
energy levels in shells and subshells, the Pauli exclusion principle, Hund’s rule
and the aufbau principle.
•Draw energy level diagrams for element and ions.
•Write the electron configuration of any element or ion and to
relate its electron configuration to its position in the periodic table.
•Know what each of the four quantum numbers n, l, m, and ms
represents.
•Identify the four quantum numbers for an electron in an atom.
•Identify the number and location of the valence electrons in an
atom.
•Identify the characteristic properties of elements in each of the s,p
and d blocks of the periodic table.
The Hellenic Market
~
Fire Water
Earth
Air
Greek Model
“To understand the very large,
we must understand the very small.”
Democritus (400 B.C.)
• Greek philosopher – “thought”
experiments
• Idea of ‘atomos’
Atomos = ‘indivisible’
• Tear up a piece of matter until
you reach the atomos.
Democritus’s model of atom
”Nothing exists but atoms and space, all else is opinion”.
Alchemy
(500 – 1400 A.D.)
Alchemical symbols for substances…
..
.
.. ..........
GOLD
SILVER
COPPER
IRON
SAND
transmutation: changing one substance into another
D
In ordinary chemistry, we cannot transmute elements.
Contributions
of alchemists:
Information about elements
- the elements mercury, sulfur, and antimony were discovered
- properties of some elements
Develop lab apparatus / procedures / experimental techniques
- alchemists learned how to prepare acids.
- developed several alloys
- new glassware
Dalton’s Atomic Theory 1805
Billiard Ball Model
1. All matter consists of tiny particles
called atoms.
2. Atoms cannot be subdivided,
created or destroyed.
3. All atoms of an element are
identical.
4. Atoms of different elements are
different from each other.
5. Atoms of different types combine is
specific ratios to form compounds.
Radioactivity (1896)
1. rays or particles produced by
unstable nuclei
a. Alpha Rays – helium nucleus
b. Beta Part. – high speed electron
c. Gamma ray – high energy x-ray
2. Discovered by Becquerel –
exposed photographic film
3. Further work by Curies
Antoine-Henri Becqu
(1852 - 1908)
Thomson’s Experiment
1897
-
voltage
source
+
vacuum tube
metal disks
Thomson’s Experiment
-
voltage
source
+
vacuum tube
metal disks
Thomson’s Experiment
ON
-
OFF
voltage
source
+
Passing an electric current makes a beam appear
to move from the negative to the positive end
Thomson’s Experiment
ON
-
OFF
voltage
source
+
Thomson’s Experiment
ON
-
voltage
source
+
+
By adding an electric field…
he found that the moving pieces were
negative.
Thomson’s Raisin Bun Model
1897
• Using cathode ray tubes, he
was able to deflect cathode
rays with an electric field.
• The rays are bent towards the
positive pole, indicating that
cathode ray particles are
negatively charged.
(electrons)
• Atom is a + sphere with –
electrons embedded.
Thomson’s Plum-Pudding or Raisin Bun
Model
Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 56
Ernest Rutherford (1871-1937)
Planetary Model of the Atom
Rutherford
PAPE
R
• Learned physics in J.J.
Thomson’ lab.
• Noticed that ‘alpha’
particles were
sometimes deflected by
something in the air.
• Gold-foil experiment
Animation by Raymond Chang – All rights reserved.
Rutherford’s Apparatus
Rutherford received the 1908 Nobel Prize in Chemistry for his pioneering work i
beam of alpha particles
radioactive
substance
circular ZnS - coated
fluorescent screen
gold foil
Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 120
Results of foil experiment if plum-pudding
had been correct.
Electrons scattered
throughout
-
+
-
positive
charges
+
+
-
+
+
-
-
+
+
-
+
Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 57
-
What he expected…
What he got…
richocheting
alpha particles
Rutherford’s
Gold Foil Experiment (1909)
Revised Theory
Interpreting the Observed Deflections
deflected particle
.
.
.
.
beam of
alpha
particles
.
.
.
.
.
.
.
.
gold foil
Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 120
.
.
.
.
.
undeflected
particles
Rutherford’s
Gold-Leaf
Experiment
Conclusions:
Atom is mostly empty space
Atom has a very small, dense,
positively charged core.
(nucleus)
Electrons float around nucleus
Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 120
Evidence for Particles
In 1886, Goldstein, using equipment similar to cathode ray tube,
discovered particles with charge equal and opposite to that of electron,
but much larger mass.
Rutherford later (1911) found these particles to be identical to hydrogen
atoms minus one electron
- named these particles protons
Chadwick (1932) discovered particles with similar mass to proton
but zero charge.
- discovered neutrons
An unsatisfactory model
for the hydrogen atom
According to classical physics, light
should be emitted as the electron
circles the nucleus. A loss of energy
would cause the electron to be drawn
closer to the nucleus and eventually
spiral into it.
Hill, Petrucci, General Chemistry An Integrated Approach 2nd Edition, page 294
Bohr’s Model
Nucleus
Electron
Orbit
Energy Levels
Niels Bohr (1913)
1. e- can only occupy certain
regions of space (orbits)
2. e- only have specific
(quantized) energy values
in an atom (energy levels)
3. e- can move from one orbit
to another by absorbing or
emitting energy, giving rise Bohr’s model could not
to characteristic spectra.
explain the spectra of
atoms heavier
than hydrogen.
Bohr Model of Atom
Increasing energy
of orbits
n=3
e-
n=2
e-
n=1
ee-
e-
e-
e-
e-
e-
e-
eA photon is emitted
with energy E = hf
The Bohr model of the atom, like many ideas in
the history of science, was at first prompted by
and later partially disproved by experimentation.
http://en.wikipedia.org/wiki/Category:Chemistry