The Gold Foil Experiment
advertisement
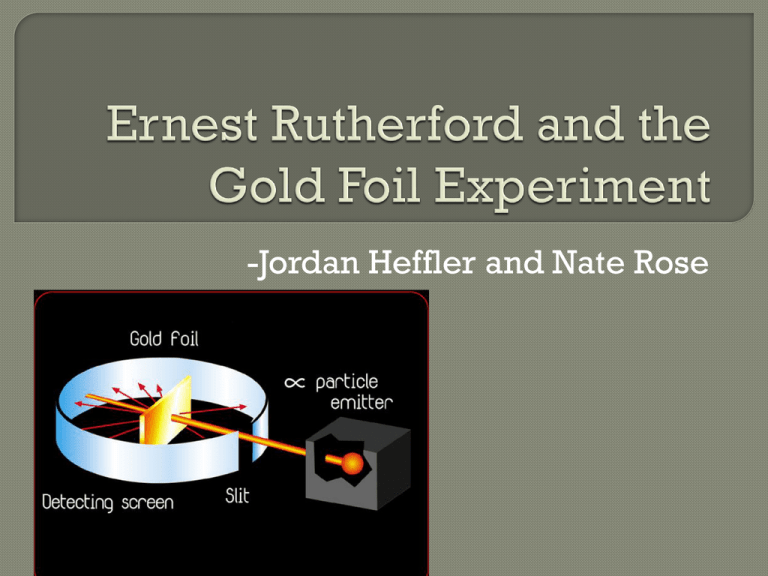
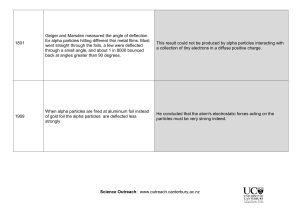
-Jordan Heffler and Nate Rose Born August 30, 1871 Born in Bridgewater, New Zealand Died October 19, 1937 Worked with Hans Geiger and Ernest Marsden on Gold Foil Experiment Famous experiment conducted at the Physical Labs of the University of Manchester Discovered the nature of the atom in 1911 and that the atom must have a hydrogen nuclei Was awarded a Noble Prize for his work in atomic disintegration in 1908 Most importantly he unleashed the power of the atom Featured on New Zealand’s 100 dollar bill and many other nations’ stamps for their appreciation of his work Was elevated to the peerage in the New Years honors list, giving him the title Lord Published 6 books Created the unit (rd) Because the experiment was to be performed in the dark, Rutherford had the experimenter sit in the lab for an hour in complete darkness, so his eyes would be immune For accuracy, Rutherford used other foils • (i.e. lead, aluminum, iron) Placed a zinc sulfide screen behind the foil so the particles that passed through could be viewed Aimed a beam of alpha particles at a piece of gold foil Used other different foils, like aluminum and iron to be more accurate Put a zinc sulfide screen behind the gold foil so the particles that passed through could be displayed According to Thomson’s theory, each alpha particle should have passed directly through the piece of gold foil with some small deflections • Alpha particles are heavy, and Thomson concluded that the charge in an atom is widely spread, so they should have mainly gone right through the foil Most models passed right through, but some were deflected at a slight angle 1 in 20,000 particles deflected off of the foil and back at the beam • An atom must contain more than empty space and electrons moving around • The atom must have a positively charged center which is much of the atom’s mass • Alpha particles are heavy and positively charged, and because some were deflected, then a big part of the atom must be heavy and also positively charged • Only a minimal amount of particles were swerved from their path when they passed through the gold foil, and even fewer bounced back • The positively charged center must be very small compared to the relative size of the atom • Overall, most of the mass of an atom is found in the small, positively charged nucleus, and the rest of the atom was mostly empty space • Electrons are grouped surrounding the nucleus • Atoms aren’t just one single particle, they’re made up of smaller subatomic particles • Proved Thomson wrong, updated the “current” model of the atom Concluded that the atom contained a dense nucleus, rather than just a lot of empty space Dingrando, Laurel. "Subatomic Particles and the Nuclear Atom." Chemistry: Matter and Change. New York, NY: Glencoe/McGraw-Hill, 2005. 92-97. Print. "Ernest Rutherford - Scientist Supreme." Ernest Rutherford - Scientist Supreme. N.p., n.d.Web. 26 Oct. 2013. <http://www.rutherford.org.nz/biography.htm>. "The Gold Foil Experiment." The Gold Foil Experiment. N.p., n.d.Web. 26 Oct. 2013. <http://myweb.usf.edu/~mhight/goldfoil.html>.