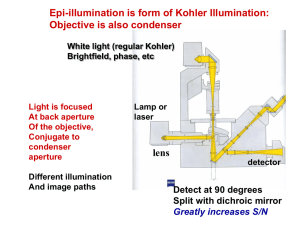
S 2 S 1 S 0
advertisement
Chemistry 2 Lecture 12 Molecular Photophysics Learning outcomes from lecture 11 • Be able to use S, D and T to label the spin multiplicity of an electronic state • Be able to describe how energy is lost after absorption by radiative transitions and non-radiative transitions • Be able to explain the “mirror symmetry” and Stokes shift of absorption and fluorescence spectra explained using a Jablonski diagram Assumed knowledge Electronic states are labelled using their spin multiplicity with singlets having all electron spins paired and triplets having two unpaired electrons. After absorption, energy is lost by radiative transitions and non-radiative transitions. Fluorescence spectra are red-shifted compared to absorption spectra but commonly have “mirror symmetry” last lecture… S1 T1 CH3 CH3 CH3 O trans-retinal CH3 CH3 (light absorber in eye) S0 Absorption spectrum of a dye 200 S2 S1 Absorbance S3 150 S3 100 S2 50 S1 0 S0 400 450 500 550 Wavelength (nm) 600 Absorption to several electronic states A cl Benzene Showing singlet and triplet absorption S3 Triplet states S2 T2 S1 T1 S0 Absorption to triplet states from single states is formally forbidden, thus very weak. Fluorescence spectrometer Key features: Two monochromators are needed - one to select a single wavelength to excite the molecule - the other to resolve the emitted wavelengths Excitation and detection occur at 90 to each other - this minimises the amount of incident light gets into the detector. Fluorescence spectrometer Two types of spectra: Fluorescence spectrum - select excitation l with mono #1 - scan mono #2 to measure fluorescence spectrum Excitation spectrum - later Real data… Absorption 500 550 Fluorescence 600 650 700 Wavelength (nm) - Fluorescence is always to longer wavelength - Stokes shift = (absorbance max) – (fluorescence max) = 50 nm here - Mirror symmetry Fluorescence spectrum f(lexc) NRD Note: the non-radiative decay (NRD) only occurs in the condensed phase, where the fluorophore can transfer vibration energy to the solvent. Stokes shift Absorption The shift between lmax(abs.) and lmax(fluor) is called the STOKES SHIFT A bigger Stokes shift will produce more dissipation of heat E nergy Energy Energy Franck-Condon Principle (in reverse) 0 1 2 3 4 5 0 1 2 3 4 5 R R E nergy Energy Energy Franck-Condon Principle (in reverse) 0 1 2 3 4 5 0 1 2 3 4 5 R R Note: If vibrational frequencies in the ground and exited state are similar, then the spectra look the same, but reversed -> the so-called “mirror symmetry” Absorption spectrum of another dye Absorbance 200 l = 400 nm Excite dye at different wavelengths 150 l = 440 nm 100 l = 550 nm 50 0 400 450 500 550 Wavelength (nm) What will emission (fluorescence) spectra look like? 600 The emission is the same! lex= 400 nm lex = 440 nm lex = 550 nm * * * 400 450 500 550 600 650 700 Wavelength (nm) 750 800 Kasha’s Law: “Emission always occurs from the lowest excited electronic state” Internal Conversion (IC) S2 NRD Emission S1 S0 “Internal conversion (IC)” is the spontaneous relaxation of an electron to a lower energy state, accompanied by a simultaneous increase of vibrational energy of the molecule. IC is a non-radiative process. Born-Oppenheimer Breakdown Terms in the Hamiltonian operator which are ignored when making the BO approximation promote transitions between states of the same energy in the molecule. Small energy gap = Good Franck-Condon factor Large energy gap = Bad Franck-Condon factor A molecule can make a non-radiative transition to an isoenergetic state. If this state can then lose vibrational energy to the solvent, it is irreversible. Fluorescence spectrometer Two types of spectra: Fluorescence spectrum - select excitation l with mono #1 - scan mono #2 to measure fluorescence spectrum Excitation spectrum - select fluorescence l with mono #2 - scan excitation l using mono #1. Under what conditions will the excitation spectrum resemble the absorption spectrum? Excitation Spectroscopy • Absorption is a DIRECT technique for measuring an electronic transition – the direct loss of transmitted light is measured. • There are other techniques to infer the absorption of light: - fluorescence excitation - phosphorescence excitation - resonant ionisation - photofragment excitation Explanation of fluorescence excitation • Because the fluorescence is the same no matter where the molecule is excited, then any (or all) fluorescence transitions can be monitored. N (fluorescence photons) N (absorbed photons) N (fluorescence photons) = ff x N (absorbed photons) • The proportionality constant, ff is called the “fluorescence quantum yield” • ff can vary between 0 and 1 • If ff is constant with l, then fluorescence excitation spectrum has the same shape (intensity vs l) as the absorption spectrum Comparison of Absorption and Excitation Spectra Note the extended p-chromophore, which is responsible for the absorption. Multiple electronic states 1500 Excitation Absorption Rhodamine 6G 0 200 250 300 350 Wavelength (nm) 400 450 When absorption excitation… Especially note this difference?? 2000 Pyrenesulfonic acid 0 200 250 300 Wavelength (nm) 350 More on Internal Conversion IC S2 Smaller energy gap IC S1 Larger energy gap IC is usually very fast between excited states and slower between S1 and the ground state. S0 Internal conversion is the relaxation of the electron to a lower level, but, accompanied by no radiation, the equivalent amount of energy is converted to vibrational energy. IC is a radiationless process Intersystem Crossing (ISC) and Phosphorescence ISC S2 T2 NRD & IC S1 ISC T1 Phosphorescence S0 “Intersystem crossing” is the flipping of an electron spin so that the molecule changes from singlet to triplet state (or vice versa). ISC is a non-radiative process and is typically 106 times slower than IC, other things being equal. More on phosphorescence Triplet states S2 Fluorescence S1 T2 T1 Phosphorescence S0 The lowest triplet is nearly always below the first excited singlet state. Therefore phosphorescence is nearly always “red shifted” (i.e. at lower energy) than fluorescence. It is formally forbidden and about 106 times slower than fluorescence. Everything together… ISC IC S2 ISC Absorption Fluorescence IC S0 T2 S1 ISC T1 Phosphorescence Phosphorescence in research Absorption spectrum S2←S0 phosphorescence S1←S0 This porphyrin molecule exhibits a huge p-system and absorbs across the visible region. The palladium metal centre promotes intersystem crossing. The molecule was synthesized in the Crossley group, and used by Schmidt’s group in solar research. Ultrafast fluorescence Using ultrafast lasers, we can observe the porphyrin in the act of fluorescing. Here, after excitation at 300 nm, fluorescence at 680 nm builds up within 3 ps but gone after only 20 ps after which the molecules which are left are all in the T1 state and then phosphoresce on the 20 ms timescale. How fast? One picosecond is one millionth of one millionth of a second. There are as many picoseconds in a second as there have been seconds since our species invented shoes, 40000 years ago. The bisporphyrin below has a very low S1 state. Ultrafast transient absorption experiments show that it does not undergo ISC, but rather IC is complete in 50ps. This experiment is 2 weeks old. S1←S0 Learning outcomes • Be able to explain Kasha’s law by describing internal conversion • Be able to define fluorescence quantum yield • Be able to describe intersystem crossing and how it leads to phosphoresence • Be able to explain why the phosphorescence occurs at lower energy (“red-shifted”) and is slower than fluorescence Next lecture • Course wrap up Week 13 homework • Electronic spectroscopy worksheet in the tutorials • Complete the practice problems at the end of the lectures • Note: ALL of the relevant past exam problems have been used as practice problems. Other questions on past papers include parts which are no longer part of the course. Practice Questions 1. The figure opposite shows the absorption, fluorescence and phosphorescence spectra of a common organic dye. Why is the phosphorescence spectrum significantly red shifted compared to the fluorescence spectrum? 2. The spectra below show the fluorescence excitation (blue) and fluorescence emission spectrum (red) of two large molecules. Explain, the following features of the spectra, using a Jablonski diagram to illustrate your answer. (a) The Stokes shift is quite different for molecules A and B. Explain how this difference arises, and give an example of what molecular property might give rise to a large Stokes shift. (b) Molecule B in particular is a nice example of “mirror symmetry” between excitation and emission spectra. How does this mirror symmetry arise? Practice Questions 3. The two spectra below show the fluorescence excitation and absorption spectra of pyrenesulfonic acid. (a) In addition to the identified electronic origin transitions, there are other peaks in the absorption spectrum, as indicated by “a)” in the figure. Using a Jablonski diagram, explain how these other peaks arise. (b) In the absorption spectrum, for the S1 and S2 transitions, the origin band is stronger than the two satellite bands marked by “a)”. In the S3 transition, the origin band is weaker than the satellite, marked by “b”. Explain, using the FranckCondon principle, how this arises. (c) Using a Jablonski diagram, describe one such process that can give rise to the observed difference in the relative intensities of the fluorescence excitation and absorption spectra at λ < 245 nm