(PFG) NMR diffusometry
advertisement

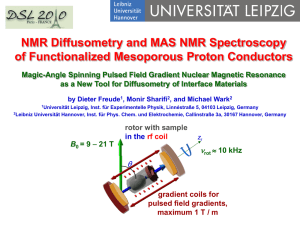
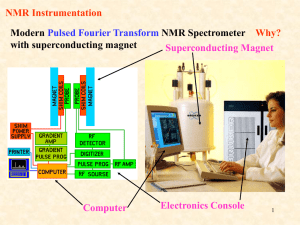
Ion and water mobility in zeolite Li-LSX studied by 1H, 6Li and 7Li NMR spectroscopy and diffusometry Investigation of the water and lithium ion mobility in a crystalline porous material by Dieter Freude, Steffen Beckert, Frank Stallmach, Jörg Kärger, Jürgen Haase Universität Leipzig Institute für Experimentelle Physik Linnéstraße 5, 04103 Leipzig, Germany rotor with sample in the rf coil B0 = 9 18 T zr rot 10 kHz θ The study was recently published in Microporous and Mesoporous Materials 172 (2013) 174–181 (15 May 2013). Reprints are available on request now or by E-mail to freude@uni-leipzig.de. gradient coils for pulsed field gradients, maximum 1 T / m for MAS, but 35 T / m for PFG NMR Pulsed field gradient (PFG) NMR diffusometry Spin recovery by Hahn echo without diffusion of nuclei: p/2 p r.f. pulse t gradient pulse gmax = 25 T / m magnetization y t d free induction Hahn echo D B0 z B0 z y y M x t D B0 1 5 4 z y 2 B0 y 5 3 1 z 4 2 3 M x PFG NMR: signal decay by diffusion of the nuclei PFG NMR diffusion measurements base on radio frequency (rf) pulse sequences. They generate a spin echo, like the Hahn echo (two pulses), or the stimulated spin echo (three pulses). At right, a sequence for alternating sine shaped gradient pulses and longitudinal eddy current delay (LED) consisting of 7 rf pulses, 4 magnetic field gradient pulses of duration d, intensity g, observation time D, and 2 eddy current quench pulses is presented. The self-diffusion coefficient D of molecules is obtained from the decay of the amplitude S of the FID in dependence on the field gradient intensity g by the equation 2 d d 4d g S S0 exp D pp S0 exp D k D 2 p High-resolution solid-state MAS NMR Fast rotation (1-60 kHz) of the sample about an axis oriented at the angle 54.7° (magic-angle) with respect to the static magnetic field zr removes all broadening effects with an rot angular dependency of B0 3 cos2 1 . 2 θ arccos 1 54.7o 3 Chemical shift anisotropy, internuclear dipolar interactions, first-order quadrupole interactions, and inhomogeneities of the magnetic susceptibility are averaged out. It results an enhancement in spectral resolution by line narrowing for solids and for soft matter. The transverse relaxation time is prolonged. MAS PFG NMR diffusometry with spectral resolution 6 5 4 3 2 d / ppm Spectral resolution is necessary for studies of systems consisting of proton species with different mobility. The spectrum shows water molecules which are located in the sodalite cages (signal at 3.8 ppm) having a small mobility and water molecules in the large cavities (signal at 4.9 ppm) having a high mobility in the hydrated zeolite Li-LSX at 373 K (observation time is 100 ms). NMR exchange spectroscopy (EXSY) p/2 p/2 t1 p/2 tmix t2 time 1 0 δ F1 / ppm 0 1 0 δ F2 / ppm 2D 6Li MAS NMR exchange spectrum of the zeolite Li-LSX obtained at 373 K with a mixing time of 1000 ms. Exchange spectroscopy is a two-dimensional NMR experiment. The free induction signal is monitored as a function of t2. Consecutive experiments give the dependence on t1. After a two-dimensional Fourier transform, we obtain cross peak intensities, which depend on the exchange between the different locations of the nuclei as a function of the mixing time tmix. MAS NMR spectroscopy and MAS PFG NMR diffusometry 1H and 6Li MAS NMR spectroscopy and 1H MAS PFG NMR diffusometry were performed in a wide-bore magnet with the external magnetic field of 17.6 Tesla. PFG NMR diffusometry 7Li PFG NMR measurements were carried out by means of the homebuilt PFG NMR spectrometer FEGRIS in the external field of 9.4 Tesla. The spectrometer is able to provide pulsed field gradient amplitudes up to gmax = 39.3 Tm-1. Microporous zeolite Li-LSX Faujasite crystallite Lithium ion Water molecule Faujasite cage The lithium form of the low-silica X type zeolite (Li-LSX) has good properties for N2/O2 separation processes, cleaning liquid nuclear waste, CO2 capture from the atmosphere, and hydrogen storage. Li-X zeolites were also used as model systems for the investigation of the electrical properties of nano-scale host/guest compounds. The commercial zeolite Li-LSX consists of crystallites with about 3 µm diameter. A zeolite Li-LSX with a diameter of about 10 µm was used for the present study. 1H and 6 Li MAS NMR spectroscopy and the results of exchange spectroscopy Signals from species which are located in the large cavities and in the sodalite cages 6.0 1H 5.0 4.0 d / ppm 3.0 MAS NMR spectrum of the hydrated Li-LSX at 373 K 1.0 2.0 0.0 d / ppm -1.0 6Li MAS NMR spectrum of the hydrated Li-LSX at 373 K 2D 1H MAS exchange spectroscopy yields for a 91% lithium exchanged zeolite Li-LSX a value of 40 ms for the mean residence time of a water molecule in the sodalite cage before jumping into the supercage. By 2D 6Li MAS NMR, the mean residence time of a lithium ion on SIc position in the sodalite cage before exchange with a SIIc position is estimated to be 150 ms. The lithium ions on SIIc positions are in much faster exchange with all cations in the supercage. 1H MAS PFG NMR and 1H PFG NMR Semi logarithmic plot of the decay of the intensity of the 1H MAS PFG NMR (open squares) and 1H PFG NMR (filled squares) signals as a function of the applied gradient strength (𝒃-value) for an observation time of 10 ms at a temperature of 373 K. 2d 4d g D p p 2 p 2 b The two-component exponential decay reflects the fast inter-crystalline diffusion and the slower intra-crystalline or intra-particle diffusion. MAS PFG NMR monitors only the less important inter-crystalline effect. The stronger gradients of the PFG NMR are necessary for the observation of the intra-effect. 7Li PFG NMR Semi logarithmic plot of the decay of the intensity of the 7Li PFG NMR signals as a function of the applied gradient strength (𝒃-value) for an observation time of 2 ms at a temperature of 373 K (circles), 423 K (triangles) and 473 K (squares) Stronger pulsed field gradients were used for this first 7Li PFG NMR observation of the self-diffusion of cations in zeolites. As usual, the larger crystallites favor the measurement of the intra-crystalline diffusion. Result Crystallites of zeolite LSX with a diameter of about 10 µm were synthesized. Crystals of this size are shown to allow the simultaneous investigation of intracrystalline mass transfer phenomena of water molecules and lithium ions in hydrated zeolite Li-LSX by NMR diffusometry. By MAS NMR spectroscopy of 1H and 6Li nuclei, the water molecules and lithium ions are found to yield two signals, a major and a minor one, which may be attributed to locations in the sodalite cages and the supercages, respectively. By 1H and 6Li exchange spectroscopy the mean residence times in the sodalite cages at 373 K are found to be about 40 ms for the water molecules and about 150 ms for the lithium cations. PFG NMR self-diffusion measurements at 373 K yield a diffusivity of about 2.0 × 10-11 m2s-1 for the lithium ions, whereas the self-diffusion coefficient for the water molecules amounts to D = 2.5 × 10-10 m2s-1. This finding is not trivial, since lithium is strongly hydrated and all water molecules belong to the hydration shells. Conclusions Crystallites of zeolite LSX with a diameter of about 10 µm were synthesized. Crystals of this size are shown to allow the simultaneous investigation of intracrystalline mass transfer phenomena of water molecules and lithium ions in hydrated zeolite Li-LSX by NMR diffusometry. By MAS NMR spectroscopy of 1H and 6Li nuclei, the water molecules and lithium ions are found to yield two signals, a major and a minor one, which may be attributed to locations in the sodalite cages and the supercages, respectively. By 1H and 6Li exchange spectroscopy, the mean residence times in the sodalite cages at 373 K are found to be about 40 ms for the water molecules and about 150 ms for the lithium cations. PFG NMR self-diffusion measurements at 373 K yield a diffusivity of about 2.0 × 10-11 m2s-1 for the lithium ions, whereas the self-diffusion coefficient for the water molecules amounts to D = 2.5 × 10-10 m2s-1. The cation diffusivity is retarded by about one order of magnitude in comparison with the water diffusivity. This notably exceeds the retardation of cation diffusion in comparison with water in free solution (publication in preparation) , reflecting the particular influence of the zeolite lattice on the guest mobility.