mechanical flotation machines
advertisement
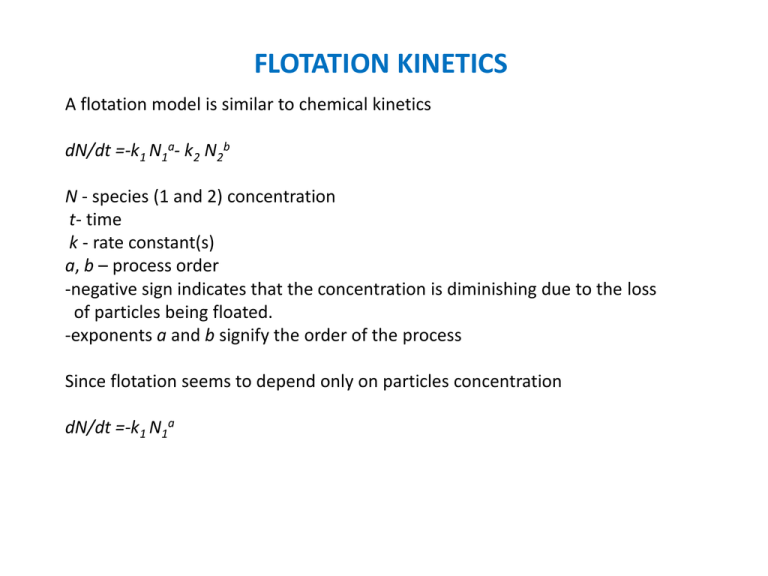
FLOTATION KINETICS
A flotation model is similar to chemical kinetics
dN/dt =-k1 N1a- k2 N2b
N - species (1 and 2) concentration
t- time
k - rate constant(s)
a, b – process order
-negative sign indicates that the concentration is diminishing due to the loss
of particles being floated.
-exponents a and b signify the order of the process
Since flotation seems to depend only on particles concentration
dN/dt =-k1 N1a
0
Flotation kinetics models
Model
Relation
= [1 – exp (–k1t)]
Classic first order
Modified first order
= {1 – 1/(k2t)[1 – exp (–k2t)]}
= [1 – 1/(1 + t/k3)]*
For reactor with ideal mixing
= k4t/(1 + k4t)*
Modified for gas–solid adsorption
= ()2 k5t/(1 + k5t)
Kinetics of second order
= {1 – [ln (1 + k6t)]/(k6t)}
Modified second order
= [1– { exp (–k7t) + (1 – ) exp(–k8t)}
Two rate constants
Distributed rate constants
* Equivalent models because k3 = 1/k4.
– flotation recovery after time t,
– maximum recovery,
– fraction of particles having lower flotation rate constant, k7,
k – flotation rate constant.
= [1 – exp(–kt) f (k, 0) dk]
Selected kinetic equations (ε – recovery of a component in separation product, εmax – maximum recovery of the
same component in separation product, k – rate constant of separation, t – separation time
Model
Formula
more
ε k t
Zeroth-order model
ε ε max 1 e
First-order model
First-order with rectangular distribution of
floatabilities
Fully mixed reactor model
-order model
2
Second-order model
Second-order model with rectangular of
floatabilites
k t
(2)
1
k t
ε ε max 1
1 e
k t
ε ε max
1
1
t
1
k
(3)
(4)
k t
ε ε max
1 k t
Improved gas/solid adsorption model
3
(1)
ε ε max
1
1
1
1 k t
2
ε
ε max
2
ε max
(5)
2
k t
1 ε max k t
1
ln 1 k t
ε ε max 1
k t
(6)
(7)
(8)
A. Bakalarz, J. Drzymala, 2013, Interrelation of the Fuerstenau upgrading curve parameters with kinetics of separation, Physicochemical
Problem of Mineral Processing, 49(2), 443-451
Flotation kinetics of the whole mass and components
40
yield of concentrate, γ, %
recovery of a component in
concentrate, ε, %
100
80
component 1
60
40
20
remaining components
30
20
sum of kinetics of
component 1 and
remaining components
10
0
0
0
10
20
separation time, min
components (recovery vs time)
30
0
10
20
separation time, min
30
product (yield vs time)
Flotation results plotted as a relationship between recovery of each component in
concentrate and separation time (a), yield of components forming concentrate vs.
separation time (b)
A. Bakalarz, J. Drzymala, 2013, Interrelation of the Fuerstenau upgrading curve parameters with kinetics of separation, Physicochemical
Problem of Mineral Processing, 49(2), 443-451
relation between flotation kinetics and upgrading curves
80
60
component 1
0
10
20
separation time, min
30
100
80
80
60
ideal upgrading
20
0
recovery of component 2 in
concentrate, ε2,c, %
ideal upgrading
100
40
recovery of component 1 in
concentrate, ε1,c, %
recovery of component 1 in
concentrate, ε1,c, %
100
Fuerstenau curve
40
20
0
0
60
20
40
60
80
100
recovery of component 2 in concentrate,
ε2,c, %
40
component 2
20
0
0
10
20
separation time, min
30
b
a
The kinetics of separation of feed
components (a) provide separation
results in the form of
the Fuerstenau upgrading curve (b).
A. Bakalarz, J. Drzymala, 2013, Interrelation of the Fuerstenau upgrading curve parameters with kinetics of separation, Physicochemical
Problem of Mineral Processing, 49(2), 443-451
ugrading curves (here Fuerstenau’s) equations based on kinetics of flotation
1, c
0
3
1
2 ,c
ε 2 ,c
ε
k' ln
1 ,c
100
ε
k ε
1 ,c
2 ,c
0
4
ε
1
1 ,c
ε 2 ,c
100
k ln
ε
1 ,c
1
ε
100 1
1 ,c
2
(1 5 k ε
)
2 ,c
100 ε 2 ,c
100
100 1
2
2
1
ε
100 1
1 ,c
100 ε 2 ,c
1 5 k ln
100
k
k' ε
ε
1 ,c
2
100
ε
1 ,c
2 ,c
100 (100 ε
2
2 ,c
)
100 ε 2 ,c
100
k ln
100 ε 2 ,c
100
100 k ln
1
7
3
2
1
ε
100 1
1 ,c
2
(1 5 k' ε
)
2 ,c
1
ε
100 1
1 ,c
100 ε 2 ,c
1 5 k' ln
100
2
ε
100 1
1 ,c
1
2
k (10 100 ε
)
2 ,c
1
100 ε
2
,c
ε
100 1
1 ,c
1
k' ε
2 ,c
1
20 (100 ε
2 ,c
)
2
9
ε
1 ,c
2
k ε
100
2 ,c
100 (100 ε
ε
2 ,c
)
1 ,c
2
100 ε 2 ,c
100
k' ln
100 ε
100 k' ln
100
2 ,c
1
c,1 recovery of component 1 in concentrate
ε
100 1
1 ,c
1
2
k ε
2
,c
1
20 (100 ε
)
2 ,c
ε
1 ,c
100 k ε
ε
2 ,c
2 ,c
( k 1) 100
13
c,2 recovery of component 2 in concentrate
Theoretical shape of the separation data in the Fuerstenau plot
recovery of component 1 in concentrate, ε1,c, %
100
100
recovery of component 1 in concentrate, ε1,c, %
recovery of component 1 in concentrate, ε1,c, %
100
80
k=1.5
k=1
k=3
60
40
k=0.5
20
80
k=2
k=1
60
k=5
k=0.4
40
20
20
40
60
80
0
recovery of component 2 in concentrate, ε2,c, %
k=0.5
60
40
k=0.02
20
k=0.005
0
0
100
80
0
0
0
k=1
20
40
60
80
recovery of component 2 in concentrate, ε2,c, %
4*
7
100
20
40
60
80
recovery of component 2 in concentrate, ε2,c, %
100
9
13
recovery of component 1 in concentrate, ε1,c, %
100
Remeber: for characterizing separation results we need
either two parameter or a law governing separation and
then you can use one parameter which can be called
selectivity as in these plots selectivity k
80
k=1
k=3
60
k=0.5
40
k=0.2
20
0
0
20
40
60
80
recovery of component 2 in concentrate, ε2,c, %
100
*for a suitable equation see previous slide
(more plots in A. Bakalarz, J. Drzymala, 2013, Interrelation of the Fuerstenau upgrading curve parameters with kinetics of separation,
Physicochemical Problem of Mineral Processing, 49(2), 443-451
An example of separation results approximation using the Fuerstenau plot
ideal upgrading
100
100
80
p la n t 3 , tria l 1
a = 1 0 2 .2 8
r
60
= a (1 0 0 - r )/(a - r )
40
20
0
80
a=100
(c o m p o n e n t 1 in p ro d u c t 1 )%
F = (89/89)
60
no upgrading
40
20
0
0
20
40
60
80
100
Polish copper ore – lab tests with xanthate
0
20
40
60
component 2 in product 2,
80
%
100
Homework
Calculate the rate constant and order of a set of yield flotation data
Microlaboratory cells
Laboratory cells
Laboratory machines
Industrial machines
Mechanical
Pneumo-mechanical
Pneumatic
Pressurized (DAF)
Other (sparged hydrocyclone, ASH)
Laboratory cells
water level
magnetic stirrer
porous glass
gas
deflector
froth
product
water level
x
stirrer
porous glass
gas
Other laboratory flotation devices
a) cylindrical cell equipped with magnetic stirrer (Fuerstenau, 1964)
b) laboratory flotation device of Partridge and Smith, 1971
flotaton product
drive
air
Laboratory Mechanobr flotation machine
Laboratory Denver flotation machine
Industrial flotation
EIMCO Product Leaflets, 2000
Flotation machines are used individually and as a group (bank)
Flotation machines are rectangular and circular
Svedala Product Handbook, 1996
Constructions and impellers of flotation machines are different
Denver
Mechanobr
Fagergreen (WEMCO-EIMCO)
DENVER
Wemco-Fagergreen (V=0.085 ÷ 85m3)
Kelly E.G., Spottiswood D.J., Introduction to mineral processing. J.Wiley& Sons, N.Jork 1985
Wemco-Fagergreen (WEMCO-EIMCO)
mechanical flotation machines
EIMCO Product Leaflets, 2000
Denver
Agitair
Metso RCS (Metso Minerals)
Outotec (Outokumpu)
X-Cell (FLSmidth Minerals)
Humbolt-Wedag
IMN Gliwice
Fragment of mechano-pneumatic flotation machine
(continueous, multi-impeller tankless Denver D-R
Wills B.A., Mineral processing
technology. Pergamon Press 1983
tailing
Pneumo-mechanic multi-tank (15m3 each)
(Aker FM – Humbold Wedag)
Humbold-Wedag Product Leaflets, 1998
Pneumo-mechanical flotation machines IMN
Maszyna przepływowa
wielowirnikowa
Maszyna
jednowirnikowa
New machines: large volume and output, saving energy
Historyczny rozwój pojemności maszyn flotacyjnych
Flotation technologies. Outotec Leaflets 2007
(Outokumpu OK-100, V= 100m3
TankCell 300
300m3
Outokumpu Oy Leaflets 2000
Flotation technologies, Outotec Oyj. Leaflets 2007
Outotec TankCell 500 (500m3)
© 2012 Outotec Oyj. www.outotec.com
RCS™ (Reactor Cell System) from 5 to 200 m3 (Metso
Minerals/Svedala)
1-radial flow of
slurry to tank
wall
2-primary slurry
stream to
benith impeller
3-secondary
recirculation
towards upper
part of tank
Basics in mineral processing. Metso
Minerals 2003
RCS™ (Reactor Cell System) from 5 to 200 m3 (Metso Minerals)
Basics in mineral processing. Metso Minerals 2003
RCS™ (Reactor Cell System) from 260 m3 (Metso Minerals)
pneumo-machanic
XCELL (FLSmidth Minerals)
XCELL™ Flotation Machines. FLSmidth Mineralss brochure 2008.
FLOTATION COLUMNS
Metso
Outotec (Outokumpu)
Jameson Cell
Imhoflot
Pneuflot (Humbolt-Wedag)
Injection Jameson Cell
Pneumatic PNEUFLOT
Pneumatic flotation with PNEUFLOT® cells HUMBOLDT WEDAG leaflet 2009
Multi-injection Imhoflot 3 (centrifugal flotation)
feed
compressed
air
air plus
suspension
feed
reagents
concentrate
tailing
feed pump
tailing pump
Pneumatic cell Imhoflot. Maelgwyn Mineral Service leaflet 4/06 Chile 2006
Injection column
Siemens
SIMINE Hybrid Flot
Metals and Mining, Siemens VAI, No. 1, 2011
Dissolved air flotation (DAF)
Dissolved air flotation (DAF)
Flotation, ZWR Polkowice