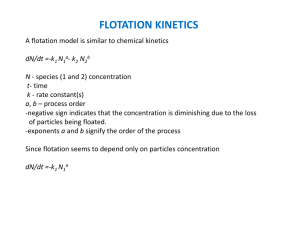
CH 2
advertisement

FLOTATION OF MINERAL MATERIALS Class 2. Native metals and sulfides A) Metals occurring in nature: iron, mercury, copper, gold, platinum B. Sulfides lead (galena, PbS) copper (chalcocite, covellite, chalcopyrite, bornite) silver (argentite) zinc (sphalerite) Class 2. Native metals and sulfides A) Metals occurring in nature: iron, mercury, copper, gold, platinum flotation with sulphydryl collectors (5 or more CH2 groups) dithiophosphates as well as xanthate + mercaptobenzothiazole, and dithiophosphate+ mercaptobenzothiazole mixtures can be used for flotation electrochemical character of adsorption of sulphydryl collectors on the surface of metals Me -5 + ++ lo g (s o lu b ility p ro d u c t) Me -15 Cd Ag Zn Cu Ni -25 Pb Au -35 Cu Hg -45 0 2 4 6 8 10 12 14 number of carbon atoms in xanthate Solubility products of metal xanthates (after Aplan and Chander, 1988) Class 2. Native metals and sulfides B. Sulfides lead (galena, PbS) copper (chalcocite, covellite, chalcopyrite, bornite) silver (argentite) zinc (sphalerite) Table 12.36. Collectors containing sulfur applied for flotation of sulfides (after Aplan i Chander, 1988) Collector type Mercaptan Dithiocarbonate (xanthate) Collectors for Formula Chemical name R–SH R–O–(C=S)–SK R–O–(C=S)–SNa flotation of sulfides potassium ethyl sodium ethyl potassium isopropyl 322 Z–9 sodium isopropyl 343 Z–11 potassium butyl – Z–7 sodium isobutyl 317 Z–14 – Z–8 sodium sec-butyl 301 Z–12 potassium amyl 355 – sodium amyl 350 Z–6 potassium sec-butyl Trithiocarbonate Xanthogen formate potassium sec-amyl – Z–5 potassium hexyl – Z–10 R–S–(C=S)–SNa Philips (Orform C0800) R–O–(C=S)–S–(C=O)–OR´ Dow R=ethyl, R´=ethyl R=izopropyl, R´=ethyl R=butyl, R´=ethyl Xanthic ester (R–O–)2(P=S)–ONa Dithiophosphate (R–O–)2(P=S)–SNa Thiocarbamate B R=amyl, R´=allyl 3302 1750 R=heksyl, R´=allyl 3461 2023 Amcy 194, 3394 AmCy (Aerofloat) Aerofloat 3477 sodium di-isoamyl Aerofloat 3501 sodium di-iso-sec-butyl Aerofloat 238 sodium di-methylamyl Aerofloat 249 cresylic acid+P 2S5 Aerofloat 15 (R–)2(P=S)–S–Na AmCy3418 R–(NH)–(C=S)–OR´ Mercaptobenzothiazole (C6H5NH2)C=S (thiocarbanilide) Na Aerofloat Aerofloat 211, 243 sodium di-izobutyl Dow Minerec N-methyl-O-isopropyl – 1703 N-methyl-O-butyl – 1331 N-methyl-O-isobutyl – 1846 N-ethyl-O-isopropyl Z–200 1661 – 1669 N-ethyl-O-isobutyl Thiourea derivatives 2048 Minerec sodium di-isopropyl Dithiophosphinate A – – sodium diethyl (R–O–)2(P=S)–SH Minerec Z–1 AmCy R–O–(C=S)–S–R’ Monothiophosphat e Manufacturer and designation Pennwalt, Philips AmCy Dow 303 Z–3 325 Z–4 AmCy Aero. 130 Seria AmCy 400 sulphides hydrophobization mechanism is complex and not well understood because there are many reactions between sulphide and sulphydryl collectors Woods (1988) and others: hydrophobization of sulfides with sulphydryl collectors results from electrochemical reactions electrons are transmitted from a collector to a sulfide mineral (anodic process), and then the electrons return to aqueous solution due to catodic reduction of oxygen anodic oxidation, mechanism a) chemisorbed xanthate Xad created from X- ion coming from aqueous solution and a metal ion crystalline structure of sulfide: X– Xad + e b) dixanthogene X2, as a result of X- ion oxidation 2X– X2 + 2e c) metal xanthate MeX2, due to reaction of X- ion with metal sulfide MS 2X– + MS MX2 + S + 2e elemental sulfur S can next form thiosulfate, sulfate(IV) or sulfate(VI) 2X– + MS + 4H2O MX2 + SO4-2 + 8H++ 8e catodic reduction of oxygen: O2 + 2H2O + 4e = 4OH- other compounds xanthogenic acid HX, hydroxyxanthates, perxanthates, disulfide carbonates, etc. are possible Hu at al., 2009 0.5 Pb(OH) 2 + S+ X 2 0.4 HPbO 2 + S+ X 2 0.3 E h , mV 0.2 PbX 2 +S HPbO 0.1 0 PbS + X 2 + S+ X - - -0.1 galena -0.2 -0.3 6 8 10 12 14 pH Eh–pH diagram for galena + ethyl xanthate. Total amount of xanthate species was 10–4 M. Formation of S is assumed (after Woods, 1988) 100 -4 2x10 M KEtX flo tatio n r eco ver y, % 80 60 -5 10 M KEtX 40 20 pyrite 0 0 2 4 6 8 pH xanthate flotation of pyrite 10 12 r ecover y dur ing fir st minut of flotation, % 100 a 80 60 40 b 20 0 -0.5 galena -0.3 -0.1 0.1 0.3 0.5 E Pt , m V Galena flotation with ethyl xanthate at pH = 8 as a function of applied potential to a platinum electrode in solution: a – galena kept in oxidizing environment before flotation, b – kept in reducing environment (Richardson, 1995; Guy and Trahar, 1985) Complications Activation Activation reaction of sphalerite with selected metal cations and calculated free enthalpy of the reactions Activation reaction ZnS +Fe2+=FeS+Zn2+ ZnS +Pb2+=PbS+Zn2+ ZnS +Cu2+=CuS+Zn2+ ZnS +2Ag+=Ag2S+Zn2+ Fe2+ FeS ZnS PbS CuS Cu2S Ag2S Conclusion: 35.2 52.5 98.1 170.7 177.5 free enthalpy, DGr0 (kJ/mol) 35.2 –17.3 –62.9 –142.3 Free enthalpy of the activation reactions for sulfides reacting with metal ions Zn2+ Pb2+ Cu2+ –35.2 –52.5 –98.1 –17.3 –62.9 17.3 –45.6 62.9 45.6 136.1 118.2 142.3 125.0 79.4 Ag+ –177,5 –142,3 –125,0 –79,4 –6,8 pyrrhotite (FeS) can be activated with all considered cations (∆Gr0 is negative), sphalerite with all cation except Fe3+, galena (PbS) only with Cu2+, and Ag+ ions. Both copper sulfides can be activated only with Ag+, while argentite (Ag2S) cannot be activated at all (∆G0f is positive). Galvanic effects 1/2O2 H2O 2OH- Bakalarz, Ph.D. thesis 2012, Rao 2004 Zn2+ S0 FeS2 2e- ZnS two sulphides sulfide mineral/cathode 2e- + 1/2O2 + H2O ↔ 2OH-aq Fe2+ OHO2/H2O e- grinding media/anode Fes → Fe(1-x)s + xFe2+aq + x2e- Bakalarz, Ph.D. thesis 2012, Greet et al., 2005 sulphide and Fe grinding medium Rest potentials (SHE) for sulfides at pH=4 (Bakalarz 2012, Ph.D. thesis) mineral pyrite marcasite chalkopyrite pyrrothite sphalerite covellite bornite pentlandite galena argentite chalcocite antymonite molybdenite heazlewoodite formula Fe S 2 (Zn, Fe)S 2 CuFeS 2 FeS ZnS CuS Cu 5FeS 4 (Fe,Ni) 9S 8 PbS Ag S 2 Cu S 2 Sb S 2 3 MoS 2 Ni S 3 2 – Dettre i Johnson, 1964, za Witika i Dobiasem, 1995 – Hiskey i Wadsworth, 1981 3 – Kocabag i Smith, 1985 4 – Bozkurt i in., 1994, za Rao, 2004 5 – Bozkurt i in., 1998 1 2 potential , mV 660 1, 630 630 1 560 1,3, 530 310 5 460 1,3 450 1, 420 400 3, 420 350 5 280 3, 400 280 1,3 440 2, 310 120 1,3 110 1,3 – 60 4 2 2 3 1 1 4 galena>bornite>shale>chalcocite >covellite>chalcopyrite chalcopyrite>bornite> covelline >shale>chalcocite, galena 100 cumulative recovery of the remaining components in the tailings, % cumulative recovery of the remaining components in the tailings, % 100 80 60 chalcocite 40 bornite chalcopyrite covellite 20 galena shale 20 60 chalcocite bornite 40 chalcopyrite covellite 20 galena organic carbon 0 0 0 80 40 60 80 100 cumulative recovery of sulfide mineral in the concentrate, % model sulfide (5%), dolomite (47.5%) and quartz (47.5%) mixture, flotation with z n-dodecane 200 g/Mg 0 20 40 60 80 100 cumulative recovery of sulfide mineral in the concentrate, % copper ore, n-dodecane 600 g/Mg, 10 min flot. (Bakalarz 2012, Ph.D. thesis Conclusion: flotation of sulfides depends on system Class 3. Oxidized minerals of non-ferrous metals cerussite (PbCO3) vanadinite (Pb5[Cl(VO4)3]) anglesite (PbSO4) malachite (CuCO3·Cu(OH)2 azurite (2CuCO3·Cu(OH)2) chrysocolla (hydrated copper silicate) tenorite (CuO) cuprite (Cu2O) smithsonite (ZnCO3) Class 3. Oxidized minerals of non-ferrous metals Approaches: 1. Sulfidization 2. Flotation using either cationic or anionic collectors (as in the case of oxide-type minerals) Sulfidization reaction -MO + S2- + 2H+ = -MS + H2O 100 1 80 re c o v e ry , % 2 60 malachite 40 20 3 0 0 10 20 dosage of amyl xanthate, mg/dm 30 40 3 Influence of conditions of flotation on recovery of malachite sulfidized with 960 mg/dm3 of Na2S·9H2O in the presence of frother (amyl alcohol 60 mg/l): 1 – flotation when after sulfidization the solution is replaced with pure aqueous, 2 – flotation after 25 minutes of air bubbling through the solution containing sulfide ions, 3 – flotation directly after sulfidization in the presence of sulfide ions (after Soto and Laskowski, 1973) also anionic and cationic collectors can be used (as for oxides and hydroxides Class 4. Oxides and hydroxides Consists of simple oxides (Fe2O3, SnO2), oxyhydroxides (AlOOH) as well as complex oxides and complex hydroxides (spinels, silicates, aluminosilicates). T able 1 2 .3 8 . Influe nce of str uctu re of silic ates o n th eir flotatio n w ith anio nic a n d cationic collectors (after M anse r, 1 9 75 ) C ollecto r S ilicate g rou p orth osilicates py rox ene a m p hibole fra m e A nio nic g ood w eek no ne no ne C atio nic satisfactory * satisfactory * g ood very g oo d * F lo tatio n d ep en d s o n p H 100 flotation r ecover y, % 80 varous minerals 60 40 albite 20 quartz 0 2 4 6 8 10 12 pH Oleate flotation of oxide and silicates Concentration - pH diagram for sodium oleate aqueous solutions showing predominance of various oleate species (Drzymala, 1990): c – activity of oleate species, mol/dm3, B (or ) – degree of binding oleate with sodium ions in associated species (number of sodium ions per one oleate ion in the associate) Comparison of pH ranges of oleate flotation of minerals as well as activated quartz and pH of existence of metal monohydroxy complexes Monohydroxy complex FeOH++ AlOH+ PbOH+ MnOH+ MgOH+ CaOH+ CuOH+ FeOH+ Range of pH at concentration> 10–7 M 0–3.9 2.1–5.9 3.2–12.4 7.6–11.6 8.4–12.5 > 8.5 5.1–8.1 4.5–12.1 pH of maximum concentration 2.7 4.3 8.7 9.5 10.5 13.1 6.5 8.7 Flotation (after a) mineral pH of maximum flotation augite 2.9 pirolusite magnesite Augite 9 10.4 11 chromite and other iron minerals 8.7, 8 pH of flotation b activated quartz 2–8* 2–8 7–13 7–13 a – Fuerstenau and Palmer (1976), b – Daellenbach and Tiemann (1964). * The participation of FeOH+ ions in widening the pH range of flotation of activated quartz activated with FeOH++ ions cannot be ruled out. Fatty acids adsorption particle oil x a b c Schematic illustration of modes of adhesion of a colloidal collector (here as an oil drop) to solid surface: a – contactless (heterocoagulation), b – contact, c – semicontact adhesion 5 80 4 60 3 40 2 zircon 20 1 0 0 0 2 4 6 8 10 initial concentration of sodium oleate, mol/dm 12 adsorption density, m ol/cm re c o v e ry , % 2 100 x 10 4 Zr[SiO4] 3 (x10 4 ) At high oleate species concentrations flotation decreases even though the oleate adsorption increases. It is assumed that it results from adsorption of hydrophilic micelles (based on data of Dixit and Biswas, 1973) 100 oleic re c o v e ry , % 80 linoleic 60 linolenic lauric 40 20 kyanit e iep 6.9 0 0 2 4 6 8 10 12 14 Al2[OSiO4] pH Kyanite flotation with 10–4 kmol/m3 of fatty acids (Choi and Oh, 1965). Applied acids: laurate (C11H23COOH), linoleic (C5H11–CH=CH–CH2–CH=CH–(CH2)7COOH), linolenic CH3–[CH2– CH=CH]3(CH2)7COOH and oleic (C17H33COOH) Adosrption of oleates on calcium minerals According to Rao and Forssberg (1991), depending on the sign of surface potential and its value for calcium minerals, the following reactions, leading to the formation of mono- and double layers of compounds, take place: on electrically neutral sites: –CaOH + –OOCR = –Ca+ –OOCR + OH– –CaOH + Na+ –OOCR + OH– = –CaO Na OOCR– + H2O –CaOH + Ca++ –OOCR + OH– = –CaO Ca OOCR– + H2O on positively charged sites: –CaOH2+ + –OOCR + OH– = –Ca+ –OOCR + H2O on negatively charged sites: –CaO– Na+ + –OOCR = –CaO Na OOCR, where < 1, –CaO– Ca++ + –OOCR = –CaO Ca OOCR, where <or = 1. AMINES Primary amine Secondary amine Tertiary amine dissociation/adsorption quaternary ammonium compounds permanetly charged R groups can be alkyl, aryl, the same or different AMINES Equilibrium constants of selected reactions, iep and CMC for dodecylamine in aqueous (after Laskowski, 1988) Reaction R–NH2 (aq)+H2O R–NH3+ (aq)+OH– R–NH2 (s) R–NH2 (aq) micellization iep lo g (a m in e c o n c e n tra tio n , k m o l/m 3 ) -1 K 4.3·10–4 2.0·10–5 CMC = 1.3·10–2 M pH = 11 iep micelle + (R-NH 3 (aq) ) n colloidal suspension -2 unstable - + -3 R-NH 2 (s) aqueous solution -4 + R-NH 3 (aq) stable -5 R-NH 2(aq) -6 5 7 9 11 13 pH Diagram of predomination of various forms of dodecylamine as a function of pH of solution (data after Laskowski, 1988) lo g (a m in e c o n c e n tra tio n , k m o l/m 3 ) -1 iep micelle + (R-NH 3 (aq) ) n colloidal suspension -2 unstable - + -3 R-NH 2 (s) aqueous solution -4 + R-NH 3 (aq) stable -5 R-NH 2(aq) -6 5 7 9 11 13 pH 100 quartz re c o v e ry , % 80 R-NH 2 precipitation 60 iep 40 5×10 -4 MC 12 H 25 -NH 2 ×HCl 20 competition of OH - 0 6 8 10 12 14 pH Relationship between quartz flotation with amine and pH. Following good flotation in alkaline solutions there is a drop in flotation as a result of precipitation of coagulating amine. At high pH an increase of flotation is caused by stable of amine suspension (after Laskowski et al., 1988) 100 100 80 60 60 20 40 -20 20 -60 0 -100 0.80 30 20 0.90 10 0 -6 10 10 -5 10 -4 10 collector concentration, kmol/m -3 10 -2 z e ta p o te n tia l, m V 40 flo ta tio n re c o v e ry quartz-dodecylamine cos a d s o rp tio n d e n s ity , m o l/m 2 x 1 0 1 1 50 3 Flotation of particles increases with increasing concentration of collector in the system and is proportional to collector adsorption and hydrophobicity caused by the adsorption. Collector adsorption is manifested by the increase of zeta potential of particles (after Fuerstenau et al., 1964 and Fuerstenau and Urbina, 1988), pH = 6–7 100 flotation recovery , % 80 QUARTZ 60 40 18 20 0 10 -0 8 10 16 -0 7 10 14 -0 6 12 10 -0 5 10 10 -0 4 8 10 -0 3 am ine concentr ation, km ol/m Amine flotation of quartz 6 10 4 -0 2 10 3 -0 1 10 00 Class 5. Sparingly soluble salts Table 12.44. Solubility product (Kr) for selected compounds at 293 K (after Barycka and Skudlarski, 1993) Compound 1 Fluoride CaF2 SrF2 MgF2 Chloride AgCl PbCl2 Bromide AgBr PbBr2 Iodide AgI PbI2 Carbonate PbCO3 ZnCO3 CaCO3 MgCO3 Hydroxide Fe(OH)3 Zn(OH)2 Mg(OH)2 Ir 2 4.0·10–11 2.5·10–9 6.5·10–9 1.8·10–10 1,7·10–5 4.6·10–13 2.8·10–5 8,3·10–17 7.1·10–9 7.2·10–14 1.7·10–11 7.2·10–9 3.5·10–8 4.5·10–37 3.3·10–17 1.2·10–11 Compound 3 sulfite BaSO4 SrSO4 CaSO4 sulfide HgS Ag2S Cu2S CuS PbS ZnS NiS CoS FeS MnS cyanide Hg2(CN)2 CuCN chromate PbCrO4 BaCrO4 CuCrO4 Ir 4 9.8·10–11 6.2·10–7 9.1·10–6 1.9·10–53 6.3·10–50 7.2·10–49 4.0·10–36 6.8·10–29 1.2·10–28 1.0·10–24 3.1·10–23 5.1·10–18 1.1·10–15 5.0·10–40 3.2·10–20 2.8·10–13 1.2·10–10 3.6·10–6 100 SDS re c ov e ry , % 80 DDA 60 fluor it e 40 NaOl 20 0 2 4 6 8 pH 10 12 14 NaOl - sodium oleate, DDA-dodecylamine, SDS,- sodium dedecyl sulfite Class 5. Sparingly soluble salts 100 chr ysocolla re c ov e ry , % 80 calcite 60 40 bastne site 20 bar ite 0 0 2 4 6 8 10 12 pH Flotation with potassium octylohydroxymate 14 the same minerals - different flotation response 100 100 80 re c o v e ry , % re c o v e ry , % 80 60 fluorit e calcite chloro apatite 40 60 ionic strength 0.002 M NaClO 4 pH = 9.5 calcite 40 fluorite 20 20 apatit e barit e 0 10 -6 10 -5 10 sodium oleate concentration, mol/dm -4 10 3 -3 0 10 -6 10 -5 10 -4 concentration of sodium oleate, mol/dm Flotation of sparingly soluble minerals with oleic acid: a – after Finkelstein (1989), natural pH, b – after Parsonage et al., (1982) 10 3 -3 Influence of different collectors and depressants on barite and fluorite flotation (table after Pradel, 2000 based on Sobieraj, 1985) Reagent Flotation of barite fluorite Collectors Alkyl sulfate Pretopon floats well at pH 8–12 reduced flotation at pH 8 Siarczanol N-2 floats well at pH 4–12 flotation at pH 6–10 Sodium dodecyl sulfate floats well at pH 4–12 cease of flotation at pH > 7 (SLS) Alkyl sulfonate gradual cease of flotation at Oleic sulfosuccinate floats well at pH 5–12 pH < 8 Streminal ML floats well at pH 5–12 floats well at pH 5–12 Sodium floats well at pH 4–12 cease of flotation at pH > 7 kerylbenzosulfonate Fatty acids Sodium oleate floats well at pH 6.5–8.5 floats well at pH 4–10 Other collectors Kamisol OC, floats well at pH 3–12 flotation at pH 3–12 cationic collector Rokanol T-16, weak collecting power weak collecting power nonionic collector Depressant Tannins Quebracho S no flotation in alkaline total cease of flotation in (+ SLS) solutions alkaline solutions Quebracho S cease of flotation at pH > 6 cease of flotation at pH > 6 (+ Pretopon G) cease of flotation in alkaline cease of flotation in alkaline Tannin (+ SLS) solutions solutions Gallic acid cease of flotation in alkaline cease of flotation in alkaline (+ SLS) solutions solutions cease of flotation in alkaline cease of flotation in alkaline Tannin D (+ SLS) solutions solutions cease of flotation in alkaline cease of flotation in alkaline Tannin M (+ SLS) solutions solutions Other depressants Dextrin no flotation in alkaline flotation at pH 6–9 (+ sodium oleate) solutions no flotation in acidic Glycerol full flotation depression at pH environment; no week (+ sodium oleate) 5–11 flotation in alkaline solutions 100 80 fluorit e re c o v e ry , % calcite + depressor 60 + depressor 40 fluorite 20 calcite 0 2 4 8 6 10 12 pH Influence of depressant (70 mg/dm3 Al2(SO4)3 and 70 mg/dm3 Na2SiO3) on flotation of fluorite and calcite mixture (dashed line) in the presence of sodium oleate (100 mg/dm3) (after Abeidu, 1973). Solid line indicates flotation in the absence of depressant Class 6. Soluble salts Sign of surface charge for selected soluble salts (after Miller et al., 1992) Salt LiF NaF KF RbF CsF LiCl NaCl KCl RbCl CsCl LiBr NaBr Surface charge sign measured predicted* + +– + + + + + + + + – – + – – + + + + + – – – – Salt KBr RbBr CsBr LiI NaI KI RbI CsI NaI·2H2O K2SO4 Na2SO4·10H2 O Na2SO4 Surface charge sign measured predicted* – + – + + + – – – – + – – + +– + –** –** –** * Predicted from the ions hydration theory for inos in crystalline lattice (Miller et al., 1992). ** Hancer et al., 1997. Soluble salts 100 flotation recove ry, % 80 Na 2 SO 4 ×10H 2 O KCl K 2 SO 4 60 40 20 0 10 NaCl Na 2 SO 4 -0 6 10 -0 5 10 -0 4 10 dodecylam ine hydr ochlor ide, km ol/m -0 3 10 3 -0 2 depressants are called blinders 100 KCl re c o v e ry , % 80 60 CMC PAM guar 40 fines 20 0 0 50 100 150 200 250 dosage of depressor, g/Mg Application of depressants for removing fines of gangue minerals during amine flotation of KCl (after Alonso and Laskowski, 1999). CMC denotes carboxymethylcellulose PAM - polyacrylamide of low molecular weight, while guar is a natural polysaccharide