Document
advertisement

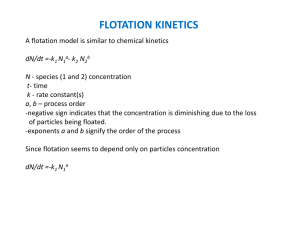
FLOTATION (Main feature - hydrophobicity) Flotation w ater gas b u b b le in tergroth h y d rop h ob ic p article h y d rop h ilic p article p łon n a (h y d rofiln a) gas bubble water particle Contact angle flotation mechanical stirring pulp bubbles particles flo ta c j a Contact angle of selected materials Advancing contact angle (degree units) mesured on polished plates (after Adamson, 1967; data from other sources denoted as *) Substance Teflon Paraffin Polystyrene Human skin Naphthalene Stearic acid Advancing contact angle 112 110 103 90 88 80 Substance sulfur graphite stibnite (Sb2S3) iodyrite (AgI) calcite (CaCO3) glass Advancing contact angle 86* 86 84 17 ~0* ~0 Methods of contact angle measurement Smoothly polished surfaces Capture bubble Adamson, 1967 heat of immersion Sessile drop Adamson, 1967 flotometry Tilted plate in liquid Hiemenz, 1986 Force of detachment Wetting of plate Drop on a tilted plate Drop shape Drop size Source* Particles Source Neumann and Good, 1979 Drzymała and Lekki, 1989a,b Drzymała, 1995; 1999a,b,c shape of border between Aveyard and Clint, 1995 phases Hiemenz, 1986 levitation Li et al., 1993 Heertjes and Kossen, 1967, Adamson, 1967 pressed disc He and Laskowski, 1992 rate of penetration of a Hiemenz, 1986 van Oss et al., 1992 thin layer of particles Ralson rate of penetration of a Washburn, 1921, Crowl i Newcombe, 1992 column of particles and Wooldridge, 1967 Ralson captured bubble for Hanning and Rutter, 1989 i Newcombe, 1992 particles Clint and Tylor, 1992; Langmuir through Aveyard et al., 1944 Huethorst and Leenaars, centrifuge 1990 capillary rise without White, 1982 probe liquid capillary rise in column of particles with probe Bartell and Whitney, 1933 liquid Methods of measurement *Additional source: Neumann i Good (1979). Hydrophobicity of materials. Contact angle is in degrees and is based on flotometric measurements Strongly hydrophobic weakly hydrophobic hydrophobic* Material Material Material 1 2 3 4 5 6 Paraffin 90+ sulfides 44–0 fluorite, CaF2 10–13 CnH2n+2 silicon carbide Teflon, C2F4 90+ 27.6 arsenic, As2O3 9.3 SiC Sulfur, S 63.2 coal Mercury, Hg 45.6 indium, In Germanium, Ge 39.7 iodyrite, AgI Silicon, Si 35.4 Talc cassiterite, SnO2 35.2 silver, Ag ilmenite, Fe molybdenite, MoS2 26–0 perovskite, CaTiO3 25 scheelite, CaWO4 9 9 hydrophilic** =0 Material 7 gypsum CaSO4·2H2O ironsilicon dolomite CaMg(CO3)2 magnetite Fe3O4 23.5 diamond, C 7.9 halite, NaCl 22– tin, Sn 7.5 brawn coal 14 14 6.4 kaolinite 6.2+ hematite, Fe2O3 boric acid, H3BO3 graphite, C 5.9+ PbJ2 gold, Au barite, BaSO4 corundum, Al2O3 HgO HgJ2 copper, Cu 6 quartz, SiO2 5 calcite, CaCO3 anhydrite, 5 CaSO4 4 bones 3.3 tourmaline 3 vegetables 3 iron, Fe amber ice, D2O * Flotometric method is able to measure contact angles smaller than 90 o. ** Other hydrophilic materials: chromite, malachite, smithsonite, azurite, rutile, zircon, mica There are different models of flotation including mechanistic, thermodynamic and probabilistic models The simplest model of flotation Thermodynamic approach Gflotation= Gfinal- Ginitial = [sg - (sc+ cg)] A for A = = 1cm2 surface area Gflotation= sg - (sl+ lg) Dupre eq. (1869) Gflotation = sg - (sl+ lg) Dupre eq. (1869) where G – thermodynamic potential (free enthalpy, Gibbs potential) sg – solid – gas interfacial energy sl – solid – liquid interfacial energy lg – liquid – gas interfacial energy unit of energy and potential G is J/m2 The Young Equation - contact angle is measured through the aqueous phase sg = sc+ cgcos Combination of the Dupre Gflotation = sg - (sl+ lg) and the Young eq. sg = sl+ lgcos provides the Dupre-Young eq. G flotation = lg(cos - 1) = 0o, cos = 1, G=0, no flotation, = 90o, cos = 0, G= - cg, hipothetically full flotation Main parameter of separation – contact angle main parameter of flotation (resulting from the simplest flotation model) contact angle ( a measure of hydrophobicity ) the Dupre-Young eq. Gflotation = lg (cos - 1) process energy main feature „electromagnetic field” of separation (flotation) system More detailed models of flotation Particle and spherical bubble G flotation cg (1 (sin ) 2/3 1 4 1/3 2 cos (1 cos ) here flotation also depends on and 1/3 ) other models take into account other parameters such as size of bubble and particle Probabilistic models for instance Schulze (1993) dNp /dt = –k Np. (first order kinetics k = Pc Pa Pstab Ptpc ZNb Rp k 2 Rb 2 1 exp c min i v 1 1 exp( 1 ) 1 exp( B0' tpc c=[2R3p(p + 1,5)/3]0,5(1,39 – 0,46 ln Rp) i = 3R2 Rp/8cbh2crit h crit = 23,3[ (1 – cos A)]0,16 F Bo’=4R2p(g + pa) +3Rp(sin2 *) f (Rb)/C C = 6 sin *sin (* + ), * 180° – /2 f(Rb) = (2 /Rb) – 2Rbg v = 0,6(Rp + Rb)2/ (laminar) v = 13(Rp + Rb)2/3/*1/3 (turbulent ) ) General models of flotation (dNp/dt = –k Np) Mao–Yoon (1997) k = PcPaPstab0.25 Sb Pc = Pa = Pstab = 1 – Pd Pd Schulze (1993) k = Pc Pa Pstab Ptpc ZNb Pc = Pa = c = [2 (p + 1.5)/3]0,5(1.39 – – 0,46 ln Rp) i = 3 Rp/8cbh2kryt, h kryt = 23.3[ (1 – cos A)]0,16 Pstab= 1 – exp(1 – 1/Bo´), * 180° – /2, Bo´ = 4 (g + pa) + +3Rp(sin2 *) f (Rb)/C C = 6 sin *sin (* + ), f (RB) = (2 /RB) – 2Rbg Ptpc 1 – exp (–v /tpc) v = 0.6(Rp + Rb)2/ laminar flow v = 13(Rp + Rb)2/3/*1/3 turbulent flow Varbanov–Forssberg– –Hallin (1993) k = EUCb = f (Pc) = RpRb E = f (Pa) E = 1 – cos Pstab taken into account in other parts of expression for k U = +2 )/(9) Cb = 3Q/(4 SV) dN/dt a Bo´ cb Cb E E1 Ek Ek´ g hkryt k Nb Np Pa Pd Pstab Ptpc Q Rp Rb Rc RF Re S Sb V Vb U Z * p A v tpc c i min * – flotation rate, – centrifugal acceleration acting on particle-bubble aggregate in a vortex of liquid, – Bond’s number, – rigidity of bubble surface (cb = 1 for rigid uneven surface, cb < 1 for movable smooth surface), – bubble concentration in pulp (number of bubbles in 1cm3 of pulp), – efficiency of attachment of particles to bubble surface (number of attached particles divided by the number of particles colliding with considered bubble), – energy barrier for adhesion of bubble and particle, – kinetic energy of collision of bubble with particle, – kinetic energy of detachment of particle and bubble (calculated from the French–Wilson Eq.), – acceleration due to gravity, – critical film thickness on surface of particle, – rate constant of flotation, – number of bubbles in flotation cell at a considered time, – number of particles subjected to flotation at a considered time, – probability of adhesion of particles to a bubble, – probability of detachment of particles from a bubble (P d = 1 – Pstab), – probability of stability of particles-bubble aggregate, – probability of formation of particle-bubble-water contact, – flow of air in flotation machine, m3/s, – particle radius, – bubble radius, – radius of the stream enabling collision of particle with bubble, – size of thin film between particle and bubble during collision, – Reynolds number, – cross section area of flotation machine, m2, – area of bubbles leaving flotation cell per time unit and per cross section area ot the cell, – rate of ascending bubble, m/s, –surface velocity of aeration defined as volume rate of aeration normalized per cross section of the flotation column, – velocity of particles in relation to velocity of bubble in the pulp, – number of particle collisions per unit time, – surface tension of aqueous solution, – quantity characterizing efficiency of collision between particles and bubbles, – dissipation energy in flotation cell, – effective density of particle in water, – dynamic viscosity, – kinematical viscosity, – pulp density, – particle density, – contact angle, – advancing contact angle, – life time of liquid vortex in flotation cell which destroys the particle–bubble aggregate, – time needed to form permanent three-phase solid-gas-liquid contact, – collision time of bubble and particle, – induction time (time of removing liquid film from particle and forming attachment), – minimal time of contact, = 3.14, = 180 – /2. FLOTATION STEPS Steps of flotation particle 1. collision r (R, r, trajectory) bubble 2. contact ( receding , contact time) R 3. adhesion ( , contact time) 4. stability ( advancing, inertia forces) 5. flotation ( buoyancy) flotacja 2 CONTACT ANGLES advancing receding equilibrium flotacja 3 receding water advancing water equlilibium flotacja 4 sg = sc+ cgcos s - = sc+ cgcos g cg liquid drop scg the Young equation vapor adsorption c x x x x x x x x x x sg x x x x x s s Contact angle determined directly from the Young equation Contact angle in degrees (Drzymała, 1994) Substance Ice Quartz Paraffin Mercury s, mN/m e, mN/m sw, mN/m cg, mN/m calculated measured* 90–120 120–135 50– 68 484 ~0 ~small 0 ~75 22–33 46 51 415 72,8 72,8 72,8 72,8 0 ~0 77– 91 95 0 0 110 43–110 FLOTATION depends on hydrophobicity hydrophobicity is measured as contact angle Electrical aspects of flotation flo ta tio n re c o v e ry , % 100 80 Ge pH iep = 2.8 naturally hydrophobic 60 40 20 0 0 2 4 6 pH 8 10 12 Electrical aspects of FLOTATION Main parametr – hydrophobicity (contact angle) which depends on energetics of three interfaces (Young eq.) sg = sl+ lgcos the Gibbs theory tells that (d )T = (–idi)T. s F ( M A ), di = RT d ln ai, o RT F a M ln (a M ) pzc adsorption chemical potential R gas constant, F Faraday cost. a activity (concentration) surface charge surface potential temperature FLOTATION main feature Hydrophobicity : contact angle field (el.-mag.) G = cg (cos –1) cg, sg, sc (cos = (sg + sc )/ cg )* / E=– , , Y = f (Y ) =–(1/R T) / lna Potential: electrical E, Chemical ln a (oraz pH, pX, ....) electrochemical E h c ,c particles bubbles *Young eq. , c collector , c frother f (activity coeff. a=fc csalt , c other flot Electrical aspects of flotation -+ water -+ moving particle + - + - + + + - -+ + -+ -+ slipping plane zeta potential -+ Structure of electrical double layer + - + - + - + + charged surface layer - + + - + - - + - + - + - particle - + + Galvani potential, 0 + - + - + + - + - + - + - + - + - + - + - + potential Y diffusive layer surface potential Y 0 potential zeta, cation concentration eg. [H + ] = [H + ] r exp ( Y /RT ) anion concentration eg. [OH - ] = [OH - ] r exp ( Y /RT ) Models of electrical double layer Helmholz (flat condenser) Gouy-Chapman (diffuse layer) Yo o -o H+ OH- Yo o d ----- H+ OH- 2 kT 0 ze quadruple layer Grahame (binding sites) Yo Yi 0 = 0 0 d triple layer Stern (rigid and diffuse layer ) o i H+ OH- d----- Yo Yi Yo Yi Yd Yd o i d---- H+ K+ OH- A- Yo Yo o i j d H+ K+ K+H2O OH- A- A-H2O Flat condenser ze sinh 0 0 2 kT Diffuse condenser Formation of electrical double layer surface charge negative positive metals ęć Me Me Me Me Me Me Me Me Me Me Me Me oxides Me O Me O O Me O Me Me O Me O salts H2O H2O Me X Me X H2O X Me X Me Me X Me X placeof particle breakage Me Me Me Me Me Me + n Me+ or Me Me Me Me Me O O MeO Me OH + n H+ or O MeO Me X X Me X + electrons MeOH2+ Me OH O + n OH- MeOH2+ +n Me+ particle /water interface or X Me Me+ X Me +n X- Formation of electrical double layer lad Me S Me S Me S Me S Me S H 2O Me S S Me OH Me SH S Me OH other S Me OH 2 Me SH + S Me OH 2 + + n OH - S Me OH Me S S Me OH +H + su rface electrical ch arge, µ C /cm 2 60 40 pzc 20 0 -20 0.001M NaCl 0.010M NaCl -40 0.100M NaCl -60 2 4 6 pH 8 10 60 zeta p oten tial, m V 40 iep 20 0 -20 0.100M NaCl 0.010M NaCl -40 0.001M NaCl -60 2 4 6 pH 8 10 interpretation: preferential adsorption of -OH groups 20 zeta potentia, mV 0 -20 D2O-ice ( n) (0.0001M) diamond ( ) -40 air ( w),Nocardia sp.( ) -60 hexadecane D2O-ice (0.001M) -80 2 4 6 8 10 12 pH zeta potential and iep for materials without functional groups Point of zero charge (pzc) and isoelectric point (iep) for selected substances in aqueous solution. A collection of pzc and iep for a great number of solids can be found in a work Parks (1965) Substance Quartz, SiO2 Oleic acid, C17H33COOH Cassiterite, SnO2 Sulfur, S Sulfides, MeS Ice, D2O Hydrocarbons, CnH2n+2 Air, O2+N2+CO2 Diamond, C Bacteria (Nocardia) Rutile, TiO2 Ilmenite, FeTiO3 Hematite, Fe2O3 Barite, BaSO4 Tenorite, CuO Dolomite, (Ca,Mg)CO3 Magnesite, MgCO3 Corundum, Al2O3 Periclase, MgO pHpzc <5 <5.5 – – 7.0 0.5 6.3 – – – 4.8–5.3 5.6 6.5–8.5 – 6.5–8.5 – pHiep 1.54 2.0 2.0–5.5 2.1 2.1–7.0 3.0–3.5 3.3 3.5 3.5 3.5 4.8–8.7 6.0–8.1 6.0–7.6 7.5 7.5 9.1 12.0 hydrophobization: with all possible chemical bondings b o il a -O-H -O d c Me S Me S S Possible modes of adsorption of collectors at particle-water interface: a – adsorption of oil on hydrophobic particle with van der Waals forces, b – adsorption of apolar molecule of collector by means of hydrogen bonding, c – adsorption of polar collector by means of simple chemical bond or electrostatic attraction, d – adsorption with formation of chelating bond. Hydrophobic part of the collector is shown in white while hydrophilic as black Not to scale. collectors non-polarne ionic chelat ing simple sulfur compounds hydrocarbons and derivatives alcohols typu S-S xanthates cationic typu O-O fatty acids typu N-N diamines typu S-N carbamines anionic amphoteric typu O-S monothiocarbonates typu O-N oximes amines merkaptans aminoalkylacids Structure of collector – CH3CH2CH2CH2 CH2CH2CH2CHCOO 2 tail (hydrophobic) head (hydrophilic) CMC a b c Collector ions can be present in aqueous solution as free ions (a), premicellar species (b) spherical micelles (c). The structures appear with increasing concentration of collector in aqueous solution. Symbol o denotes ion appositively charged to the collector ion 100 rec o ve ry, % 80 CMC CMC SOS SDS 60 40 20 alumina 0 -7 10 10 -6 10 -5 10 -4 10 -3 collector concentration, kmol/m 10 -2 10 -1 3 An example of lack of correlation between CMC and flotation (after Freund and Dobias, 1995). SOS – sodium octyl sulfate, SDS – sodium dodecyl sulfate a b c Adsorption of ionic collector on the surface of particle with the formation of hemimicelle (a), monolayer (b) and a second layer leading to hydrophilicity (c) Collector Chain length* ethyl butyl Dithiocarbaminate 60 77 Mercaptane 60 74 Xanthate 60 74 Dithiophosphate 59 76 Trithiocarbonate 61 74 Monothiocarbonate 61 73 Maximum contact angle for different collectors with ethyl and butyl chain. After Gaudin, 1963 * For methyl chain (C1) 50°, propyl (C3) 68°, C5 78°, C6 81°, for greater about 90°, and for C16 98° (Aplan and Chander, 1988). collector renders the surface hydrophobic gas collector particle 100 100 80 60 60 20 40 -20 20 -60 0 -100 0.80 30 20 0.90 10 0 -6 10 10 -5 10 -4 10 collector concentration, kmol/m -3 10 -2 ze ta p o te n tia l, m V 40 flo ta tio n re c o v e ry quartz-dodecylamine cos a d s o rp tio n d e n s ity , m o l/m 2 x 1 0 1 1 50 3 Flotation of particles increases with increasing concentration of collector in the system and is proportional to collector adsorption and hydrophobicity caused by the adsorption. Collector adsorption is manifested by the increase of zeta potential of particles (after Fuerstenau et al., 1964 and Fuerstenau and Urbina, 1988), pH = 6–7 Which interface is responsible for hydrophobicity increase with collector addition? 450 90 425 H2O 400 Hg/ air 50 375 Hg/H 2 O 350 surface tenion, m N /m contact angle, degree 70 te n s io n , m N /m s u rfa c e o r in te rfa c ia l 30 -7 -6 -5 -4 -3 log (collector concentration, mol/dm -2 -1 3) Dependence of contact angle and the state of mercury at interfaces on concentration of collector Data by Smolders (1961) taken from various sources: contact angle in dodecyl sulfate solution and H2O (dodecyl sulfate) (Leja, 1982), Hg /H2O(decyl sulfonate) and Hg (decyl sulfonate) (de Bruyn i Agar, 1962)) collector sg , sc cg co n tact an g le , initial contact angle 0 cg s g s c cos = ------------- sc sg cg sc cg sg electrolytes (pH regulators and salts) modifying reagents hydrophilization reagents Flotation is influenced by reagents modifying the interfaces. Collectors strongly change the solidgas, surfactants water–gas, and electrolytes (pH reagents and salts) solid–water interfaces. The extent of modification is expressed by the height of symbol Flotation vs collector dose 100 c o n t a c t a n g le , , d e g r e e c o n ta c t a n g l e , d e g re e 80 60 40 galena 20 80 pH = 7 pH = 9 60 pH = 10 40 pH = 10.5 20 hematite - NaOl 0 0 2 4 6 8 concentration of potassium ethyl xanthate, g/m 10 3 0 10 -0 7 10 -0 6 10 -0 5 10 -0 4 concentration of sodium oleate, kmol/m -0 3 10 3 Influence of pH and iep on flotation for various collectors 100 100 goethite 80 re c o v e ry , % re c o v e ry , % 80 kyanit (collector : oleic acid) 60 40 ie p 6.9 20 collector RSO 4 Na 60 ie p 6,7 40 20 0 0 0 2 4 6 8 10 12 2 14 4 6 100 8 10 12 pH pH 100 coal 80 quar tz 80 (collector: tridecane) (collector: octylphenylpolyethoksyethanol) re c o v e ry , % re c o v e ry , % collector RNH 3 Cl 60 40 ie p 2 3 20 60 40 ie p 2 3 20 0 0 2 4 6 8 pH 10 12 2 4 6 8 pH 10 12 Collector as chemicals Ionic collectors Collector Example Cationic Primary amines* R–NH2 n-dodecylamine C12H25–NH2 or C12H25–NH2·HCl (C12H25–N+H3Cl–) Secondary amines* R–(R´)N–H di-n-amylamine, (C5H11)2–NH Tertiary amines* R–(R´)N–R´´ tri-n-amylamine, (C5H11)3–N R' Cl N+ Quarternary ammonium salts R Diamines and triamines R'' - R'' diamine R–NH–(CH2)x–NH2 C lN+ Pyridinium salts R CH2-CH2 Morpholinium salts + O Cl R NH CH2-CH2 R' R'' S + Sulfonium salts R Cl Anionic Mercaptans, R–SH Due to unpleasant smell mercaptans are not used by industry amphoteric N-dodecyl-2-aminopropionic acid C12H25–N+H2–(CH2)2–COO– other C12H25–NH–CH2–COONa, C12H25–N(CH2–COONa)2, R–(CH3)2N+–CH2– COO– (alkyl betaines) Selected chelating collectors. Type O–O. Based on Nagaraj, 1988 Collector Formula Carbonic acid derivatives R–COOH (fatty acids) Sulfuric acid derivatives R–O–SO3H (sulfates) Sulfuric acid derivatives R–SO3H (sulfonates) Phosphoric acid derivatives (RO)2–P(O)–OH (phosphates) Phosphonic acid derivatives (phosphonates, diphosphonates) Phosphinic acid derivatives (phosphinate) Nitrosophenylhydroxylamine (ammonium salt) Salicylaldehyde Nitrosonaphthols Nitrosophenols Organic dyes Hydroxamic acids (RO)–(R)P(O)–OH R–(PO3H2)2 (R)2–P(O)–OH (Ar–N(O–)–N=O)NH4 OH–Ar–CHO ON–nA–OH R–(OH)Ar(OH)–NO R–CO–NH–OH Example oleic, linoleic, linolenic, stearic acids dodecyl sulfate dodecyl sulfonates dialkyl phosphoric acid dialkyl phosphonic acid Flotol-7,9 (1-hydroxyalkylidene-1,1diphosphonic acid) dialkyl phosphinic acid Cupferron Salicylaldehyde -nitroso--naphthol, -nitroso-naphthol nitroso alkyl resorcinol alizarin and derivatives Benzohydroxamic acid, potassium octylhydroxamate, IM-50 (C7-C9) .Selected chelating collectors. Type S – S Collector Based on Nagaraj, 1988 Formula S Dithiocarbonates (xanthate) - C - OS Trithiocarbonates (tioxanthate) Example Potassium ethyl xanthate (R–OCSSK) S - C - SS S Dithiophosphates - P(OR) 2 Aerofloat ((RO)2 P(=S)–SK) S S Dithiophosphinates - PR 2 Aerofins S S Dithiocarbamates - C - NR2 S Sodium diethyldithiocarbamate Selected chelating collectors. Type O–N. Based on Nagaraj, 1988 collector formula Example - benzoin oxime CH Oximes OH C CH N OH C OH N OH LIX65N C9 H1 9 Hydroxyoximes (LIX series) C OH N OH 8- hydroxyquinoline and derivatives N OH 8- hydroxyquinoline (oxine) Selected chelating collectors. Type S - N Collector Based on Nagaraj, 1988 Formula C S C SH C N (flotagen) R C N C SH N S C NH C SH N C S Mercaptobenzothiazols Mercaptothiodiazoles Thiotertrahydroglyoxaline Mono-and dithiocarbamates C4H9O NH C N Phenylthiourea S C N H H C2H5 Apolar collectors Collector Hydrocarbons and derivatives Sulfur compounds Alcohol and derivatives Example fuel oil, naphtha, heptane, benzene, halogen derivatives of hydrocarbons dixantogen (R–O–C(=S)–S–)2 formic xanthate R–O–C(=S)–S–C(=O)–O–R´, alkyl disulfides R–S–S–R alkylfenyl(polyethoxy) alcohols (Triton, Tergitol, Brij), alkylphenols, higher alcohols Collectors ANIONIC Alkyl mercaptan R-S-H Alkyl dithiocarbonate (xanthate) R-O-CS-S-Na Dialkyl disufide (dixanthogen) R-O-CS-S-S-CS-O-R Xanthogen formates R-O-CS-S-CO-O-R’ Dialkyl dithiocarbamate RR-N-CS-S-Na Dialkyl dithiophosphate RO(RO)-PS-S-Na Carboxylate (fatty acids) R-CO-O-H Alkyl sulfate R-O-SO3H Alkyl sulfonate R-SO3H CATIONIC Amine R-NH2, RR-NH, RRR-N Quaternary Amine Cl- R+RRR-N FLOTATION METHODS Foam separation Minerals flotation Flotation with soluble collectors Emulsion flotation Froth flotation Precipitate flotation Agglomerative flotation Frothless separation Ions flotation Carrier flotation Methods of flotation Microorganisms flotation and gamma flotation (flotation in water mixed with soluble organic liquid) 1 100 co s 0,75 75 cos 0,5 0,25 50 25 surface tension of wetting 0 0 0 20 40 surface tension, 60 flo ta tio n re c o ve ry , , % 80 wg , mN/m Typical shape of the cos = f (surface tension of liquid) relationship also called the Zisman plot, and flotation of naturally hydrophobic materials FLOTATION classification upgrading splitting sorting, etc. evaluation analysis c th cs s ic s ni P1 phy ec ha od m m h er ot er yn am ic s delineation Cs x1 chemistry of flotation x2 x4 x3 xn Cp P2