Kinetics
advertisement

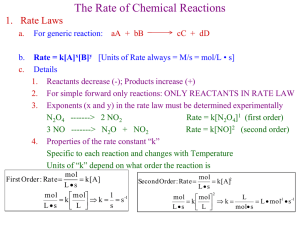
KINETICS Chapter 14 14.1 Factors that Affect Reaction Rates Chemical kinetics is the study of how fast chemical reactions occur. –Generally, the more frequently collisions between reaction particles occur, the faster the reaction. There are several important factors which affect rates of reactions: Physical state of the reactants – Homogeneous mixtures react faster than heterogeneous mixtures. – When reactants are in different phases: more surface area faster reaction Concentration of the reactants – more reactant molecules so they collide more frequently faster reaction Temperature of the reaction – higher temperature molecules move faster and collide more frequently faster reaction Presence or absence of a catalyst – use a catalyst usually changes mechanism 14.2 Reaction Rates • The speed of a reaction is defined as the change that occurs per unit time. • It is often determined by measuring the change in concentration of a reactant or product with time [M/sec] (could also use change in moles but if volume is constant [most reactions], then molarity and moles are directly proportional – concentration is easier to directly measure than moles. Pressure is sometimes used for gases.) • The speed of the chemical reaction is its reaction rate. • • • • • • Suppose A reacts to form B. AB Let us begin with [A] = 1.00 M At t = 0 (time zero) there is 1.00 M A and no B present. At t = 20 sec, there is 0.54 M A and 0.46 M B. At t = 40 sec, there is 0.30 M A and 0.70 M B. We can uses this information to find the average rate of the reaction. • For the reaction A B, there are 2 ways of measuring rate: • The rate of appearance of product B (i.e., change in concentration of B per unit time) as in the preceding example. Avg Rate = Δ (Conc. B) (Conc. of B at t = 20s)-(Conc. of B at t = 0s) = Δt 20s - 0s 0.46M - 0.00 M M = 0.023 20 s -0 s s • The rate of disappearance of reactant A (i.e., the change in concentration of A per unit time): M - Δ(conc. of A) - (0.54 M - 1.00 M) Avg Rate = = 0.023 s Δt (20 - 0 s) Avg Rate = • Note the negative sign! – Reaction rates are always positive! Change of Rate with Time •The average rate generally decreases with time •The rate at any instant in time is called the instantaneous rate. • It is the slope of the straight line tangent to the curve at that instant. • Instantaneous rate is different from average rate. • We usually call the "instantaneous rate" the rate. Example: Using the data in table above, calculate the average rate of disappearance of C4H9Cl over the time interval from 50.0 to 150.0 seconds. (b) Using the figure, estimate the instantaneous rate of disappearance of C4H9Cl at t = 0 (the initial rate). [C4 H 9Cl ] Average rate t (0.0741 - 0.0905) M 1.64 x 10-4 M / s (150.0 - 50.0) s (b) the initial rate is given by the slope of the dashed line (y / x) pick two points on the tangent line: (0, .100) and (200, .060) (0.060 - 0.100) M Rate 2.0 x 104 M / s (200 - 0) s Reaction Rates and Stoichiometry • For the reaction: C4H9Cl(aq) + H2O(l) C4H9OH(aq) + HCl(aq) • The rate of appearance of C4H9OH must equal the rate of disappearance of C4H9Cl. Δ C4H9Cl Δ C4H9OH Rate = = Δt Δt What if the stoichiometric relationships are not oneto-one? For the reaction: 2HI(g) H2(g) + I2(g) The rate may be expressed as: Δ H2 Δ I2 1 Δ HI Rate = = = 2 Δt Δt Δt We can generalize this equation a bit. For the reaction: aA + bB cC + dD The rate may be expressed as: Δ A Δ C Δ B Δ D Rate = = = = aΔt bΔt cΔt dΔt The isomerization of methyl isonitrile CH3NC to acetonitrile, CH3CN, was studied in the gas phase at 215oC and the following data was obtained: Time (s) 0 2000 5000 8000 12000 15000 [CH3NC] (M) 0.0165 0.0110 0.00591 0.00314 0.00137 0.00074 (a) Calculate the average rate of reaction in M/s, for the time interval between each measurement. (a) Time (sec) 0 2000 5000 8000 12000 15000 Time Interval (Δt) (sec) 2000 3000 3000 4000 3000 [CH3NC] (M) 0.0165 0.0110 0.00591 0.00314 0.00137 0.00074 M - 0.0055 - 0.0051 - 0.00277 - 0.00177 - 0.00063 Rate (ΔM/ Δt) 2.8 10-6 - ( - 0.0055 M / 2000 s) 1.7 10-6 .923 10-6 .443 10-6 .21 10-6 Consider the hypothetical reaction: A(aq) B(aq). A flask is charged with 0.065 mol of A and allowed to react to form B in a total volume of 100.0 mL. The following data are collected: Time (min) 0 10 20 30 40 Mol of A 0.065 0.051 0.042 0.036 0.031 (a) Calculate the number of moles of B at each time in the table, assuming there are no molecules of B at time zero (b) Calculate the average rate of disappearance of A for each 10 min interval, in units of M/s. (c) Between t = 10 min and t = 30 min, what is the average rate of appearance of B in units of M/s? Assume the volume of the solution is constant. Time(min) 0 10 20 30 40 (c) Mol A 0.065 0.051 0.042 0.036 0.031 (a) Mol B 0 0.014 0.023 0.029 0.034 [A] 0.65 0.51 0.42 0.36 0.31 [A] (b) Rate -( [A]/time) - 0.14 - 0.09 - 0.06 - 0.05 2.3 10-4 2 10-4 1 10-4 0.8 10-4 M B (0.029 - 0.014 mol)/0.100 L 1 min = x = 1.3 x 10-4 M/s t (30-10) min 60 s - ( - 0.14 M / 600 s) For each of the following gas-phase reactions, indicate how the rate of disappearance of each reactant is related to the rate of appearance of each product: (a) H2O2(g) H2(g) + O2(g) (b) 2 N2O(g) 2 N2(g) + O2(g) (c) N2(g) + 3 H2(g) 2 NH3(g) (a) ∆ 𝐻2 𝑂2 − ∆𝑡 (b) ∆ 𝑁2 𝑂 − 2∆𝑡 (c) ∆ 𝑁2 − ∆𝑡 = ∆ 𝐻2 ∆𝑡 = ∆ 𝑁2 2∆𝑡 = ∆ 𝑁2 − 3∆𝑡 = ∆ 𝑂2 ∆𝑡 = ∆ 𝑂2 ∆𝑡 = ∆ 𝑁𝐻3 2∆𝑡 Consider the combustion of H2(g), 2 H2(g) + O2(g) 2 H2O(g). If hydrogen is burning at the rate of 0.85 mol/s, what is the rate of consumption of oxygen? What is the rate of formation of water vapor? [H 2 O] [H 2 ] [O 2 ] ==2t 2t t - [H2 ] H2 is burning, = 0.85 mol/s t [O 2 ] [H 2 ] O 2 is consumed, = = t 2t H2O is produced, 1 x (0.85 mol/s) = 0.43 mol/s 2 1 [H2 O] 1 [H2 ] = = 0.85 mol/s 2 t 2 t The reaction 2 NO(g) + Cl2(g) 2 NOCl(g) is carried out in a closed vessel. If the partial pressure of NO is decreasing at the rate of 23 torr/min, what is the rate of change of the total pressure in the vessel? The change in total pressure is the sum of the changes of each partial pressure. NO and Cl2 are disappearing and NOCl is appearing. P NO = 23 𝑡𝑜𝑟𝑟/𝑚𝑖𝑛 − t − PNO = − ∆𝑃𝐶𝑙 = ∆𝑃𝑁𝑂𝐶𝑙 ∆𝑡 2∆𝑡 2t ΔPNO/Δt = -23 torr/min (given) ΔPCl /Δt = ½ (ΔPNO/Δt ) = ½ (-23) = -11.5 torr/min ΔPNOCl /Δt = - ΔPNO/Δt = -(-23) = +23 torr/min PTOT = -23 – 11.5 + 23 = -11.5 = -12 torr/min Pressure is decreasing at a rate of 12 torr/min 14.3 Concentration and Rate In general, rates: • Increase when reactant concentration is increased. • Decrease when reactant concentration is decreased. • We often examine the effect of concentration on reaction rate by measuring the way in which reaction rate at the beginning of a reaction depends on different starting conditions. • Consider the reaction: NH4+(aq) + NO2– (aq) N2(g) + 2H2O(l) • We measure initial reaction rates. • The initial rate is the instantaneous rate at time t = 0. • We find this at various initial concentrations of each reactant. • As [NH4+] doubles with [NO2–] constant the rate doubles. – We conclude the rate is proportional to [NH4+]. • As [NO2–] doubles with [NH4+] constant the rate doubles – We conclude that the rate is proportional to [NO2–]. Rate Law • The overall concentration dependence of reaction rate is given in a rate law or rate expression. • For a general reaction the rate law is: Rate = k [A]m [B]n The proportionality constant k is called the rate constant. Once we have determined the rate law and the rate constant, we can use them to calculate initial reaction rates under any set of initial concentrations. Exponents in the Rate Law The exponents m and n are called reaction orders The rate is “m” order in A The rate is “n” order in B We commonly encounter reaction orders of 0, 1 or 2. But, fractional or negative values are possible. The overall reaction order is the sum of the reaction orders. The overall order of a reaction is m + n + …. Note that reaction orders must be determined experimentally. They DO NOT necessarily correspond to the stoichiometric coefficients in the balanced chemical equation! (but they can and sometimes do) Using Initial Rates to Determine Rate Laws • To determine the rate law, we observe the effect of changing initial concentrations. • A reaction is nth order if multiplying the concentration by x causes a xn increase in rate. x increase in concentration = xn increase in rate (n is the order) For our example – the concentration was multiplied by 2 and the rate was also multiplied by 2 (21) so the rate is 1st order with respect to both NH4+(aq) and NO2– (aq) 2 (increase in concentration) = 21 (increase in rate) (1st order) • Most Common Scenarios: Another way to look at order of reaction vs. change in concentration: Zero order rxn: change in concentration = no change in rate 1st order rxn: change in concentration = change in rate 2nd order rxn: change in concentration = change in rate is the square of change in concentration change in conc. of reactant doubled change in rate no change - rate is multiplied by 1 or 20 doubled doubled - rate is multiplied by 2 or 21 doubled quadrupled - rate is multiplied by 4 or 22 tripled no change - rate is multiplied by 1 or 30 tripled tripled - rate is multiplied by 3 or 31 tripled rate is multiplied by 9 or 32 order 0 1 2 0 1 2 • For the reaction in our example: NH4+(aq) + NO2– (aq) N2(g) + 2H2O(l) The rate law is: Rate = k [NH4+] [ NO2–] • The reaction is said to be first order in [NH4+], first order in [NO2–], and second order overall. Units of Rate Constants • Units of the rate constant depend on the overall reaction order. • Second order overall: • Rate = k [A]2 Units of rate = (units of rate constant)(units of conc.)2 Ms (Units of rate) -1 -1 = M s Units of rate constant = = 2 2 M (Units of conc.) • First order overall: • Rate = k [A] Units of rate = (units of rate constant)(units of conc.)1 Ms (Units of rate) = Units of rate constant = = 1 1 M (Units of conc.) s-1 • Note that the rate, not the rate constant, depends on concentration. • The rate constant IS affected by temperature and by the presence of a catalyst (more on this later). Examples of rate laws What are the individual and overall reaction orders for the reactions described in the following equations: (1) 2 N2O5(g) 4 NO2(g) + O2(g) Rate = k [N2O5] (2) CHCl3(g) + Cl2(g) CCl4(g) + HCl (g) Rate = k [CHCl3][Cl2]1/2 • Reaction (1) is first order in N2O5 and first order overall. • Reaction (2) is first order in CHCl3 and ½ order in Cl2 and 3/2 order overall (add the exponents). A reaction A + B C, obeys the following rate law: rate = k [B]2. (a) if [A] is doubled, how will the rate change? Will the rate constant change? (b) What are the reaction orders for A and B? What is the overall reaction order? (c) What are the units of the rate constant? (a) if [A] is doubled, there will be no change in the rate or the rate constant (b) the reaction is zero order in A, second order in B and second order overall (c) rate = k [B]2 units of rate = (units of k)(conc. units) 2 rate units units of k = (conc. units) 2 M 1 s units of k = 2 = = M 1s-1 M Ms The decomposition of N2O5 in carbon tetrachloride proceeds as follows: 2 N2O5 2 NO2 + O2. The rate law is first order in N2O5. At 64oC, the rate constant is 4.82 10-3 s-1. (a) Write the rate law for the reaction. (b) What is the rate of reaction when [N2O5] = 0.0240 M? (c) What happens to the rate when the concentration of N2O5 is doubled to 0.0480 M? (a) rate = k [N2O5] = (4.82 10-3 s-1) [N2O5] (b) rate = (4.82 10-3 s-1) (0.0240 M) = 1.16 10-4 M/s (c) one way: rate = 4.82 10-3 s-1 (0.0480 M) = 2.31 10-4 M/s quicker way: We know that the rxn is 1st order in N2O5, so doubling the conc. will double the rate (a) (b) (c) Consider the following reaction: CH3Br + OH-1 CH3OH + Br-1 . The rate law for this reaction is first order in CH3Br and first order in OH-1. When [CH3Br] is 0.0050 M and [OH-1] is 0.050 M, the reaction rate at 298 K is 0.0432 M/s. What is the value of the rate constant? What are the units of the rate constant? What would happen to the rate if the concentration of OH-1 were tripled? (a,b) rate = k [CH3Br] at 298 K, k = (c) [OH-1] rate k = [CH3 Br][OH-1 ] 0.0432 M/s = 1.7 x 102 M-1s-1 (0.0050 M)(0.050 M) Since the rate law is first order in [OH-1], if [OH-1] is tripled, the rate triples. The iodide ion reacts with hypochlorite ion (the active ingredient in bleach) in the following way: OCl-1 + I-1 OI-1 + Cl-1. This rapid reaction gives the following rate data: Experiment [OCl-1] (M) [I-1] (M) Rate (M/s) 1 0.0015 0.0015 1.36 10-4 2 0.0030 0.0015 2.72 10-4 3 0.0015 0.0030 2.72 10-4 (a) What is the rate law for the reaction? (b) What is the value of the rate constant? (c) Calculate the rate when [OCl-1] = 0.0020 M and [I-1] = 0.00050 M (a) from exp. 1&2, doubling [OCl-1] while keeping [I-1] constant doubles the rate – rxn is 1st order in OCl-1 from exp. 1&3, doubling [I-1] while keeping [OCl-1] constant doubles the rate – rxn is 1st order in I-1 rate = k [OCl-1][I-1] (b) solving the rate law for k and using the data of experiment 1: rate 1.36 x 10-4 M/s -1 -1 k= = = 60 M s -1 -1 [OCl ][I ] (0.0015 M)(0.0015 M) (c) using the rate law (from a) and the value of k (from b): rate = (60 M-1s-1 ) (0.0020 M) (0.00050 M) = 6.0 x 10-5 M/s (a) (b) (c) (d) (a) (b) Consider the gas-phase reaction between nitric oxide and bromine at 273oC: 2 NO + Br2 2 NOBr. The following data for the initial rate of NOBr were obtained: Experiment [NO] (M) [Br2] (M) Initial Rate (M/s) 1 0.10 0.20 24 2 0.25 0.20 150 3 0.10 0.50 60 4 0.35 0.50 735 Determine the rate law. Calculate the value of the rate constant for the appearance of NOBr. How is the rate of appearance of NOBr related to the rate of disappearance of Br2? What is the rate of disappearance of Br2 when [NO] = 0.075 M and [Br2] = 0.25? From exp. 1&2, multiplying [NO] by 2.5 multiplies the rate by 2.52 – rxn is 2nd order in NO From exp. 1&3, multiplying [Br2] by 2.5 multiplies the rate by 2.5 – rxn is 1st order in Br2 The rate law for the appearance of NOBr is : rate = k [NO]2[Br2] from experiment 1, k = 24 M/s 4 -2 -1 = 1.2 x 10 M s 2 (0.10 M) (0.20 M) (a) (b) (c) (d) Consider the gas-phase reaction between nitric oxide and bromine at 273oC: 2 NO + Br2 2 NOBr. The following data for the initial rate of NOBr were obtained: Experiment [NO] (M) [Br2] (M) Initial Rate (M/s) 1 0.10 0.20 24 2 0.25 0.20 150 3 0.10 0.50 60 4 0.35 0.50 735 Determine the rate law. Calculate the value of the rate constant for the appearance of NOBr. How is the rate of appearance of NOBr related to the rate of disappearance of Br2? What is the rate of disappearance of Br2 when [NO] = 0.075 M and [Br2] = 0.25? (c) [Br2 ] [NOBr] =2 t t (d) The rate law for the appearance of NOBr is : rate = k [NO]2[Br2] The rate of disappearance of Br2 is ½ the rate of appearance of NOBr. [Br2 ] 1 = k [NO]2[Br2 ] t 2 1 (1.2 x 104 M -2s-1 ) (0.075 M) 2 (0.25 M) = 8.4 M/s 2 Consider the reaction of peroxydisulfate ion, S2O8-2 , with iodide ion, I-1 , in aqueous solution: S2O8-2 + 3 I-1 2 SO4-2 + I3-1 At a particular temperature, the rate of disappearance of S2O8-2 varies with reactant concentrations in the following manner: Experiment [S2O8-2] (M) [I-1] (M) Initial Rate (M/s) 1 0.018 0.036 2.6 10-6 2 0.027 0.036 3.9 10-6 3 0.036 0.054 7.8 10-6 4 0.050 0.072 1.4 10-5 (a) Determine the rate law for the reaction. (a) from experiments 1 and 2, increasing [S2O8-2] by 1.5 increases the rate by 1.5 so [S2O8-2] is 1st order Experiment [S2O8-2] (M) [I-1] (M) Initial Rate (M/s) 1 0.018 0.036 2.6 10-6 2 0.027 0.036 3.9 10-6 3 0.036 0.054 7.8 10-6 4 0.050 0.072 1.4 10-5 ALTERNATE SOLUTION In exp 1 and 2, keeping [I-1] constant and increasing [S2O8-2] by 1.5 increases the rate by 1.5 meaning that the reaction is first order in [S2O8-2] So we know that the rate law is: rate = k [S2O8-2] [I-1]x and we need to find x Looking at exp 1 and 3, the rate inc by a factor of 3 or Rateexp 3 / Rateexp1 = 3 Rate 3 k [S2O8-2] [I-1]x k [.036] [.054]x 2 [.054]x -------- = -------------------- = -------------------- = ------------- = 3 Rate 1 k [S2O8-2] [I-1]x k [.018] [.036]x [.036]x [.054]x 3 -------- = ----- (or 1.5) [.036]x 2 (1.5)x = 1.5 x = 1, so the reaction is 1st order in [I-1] rate = k [S2O8-2] [I-1] Kinetics Part 2 14.4 • Goal: Convert the rate law into a convenient equation that gives concentration as a function of time. First-Order Reactions • Rate must depend on only one reactant and be raised to the 1st power. • For a first-order reaction, the rate doubles as the concentration of a reactant doubles. Rate = k[A] A Rate t A Rate k A t (slope= −k) ln[A]t After some calculus……… Time (s) ln A kt ln A t y = mx + b 0 A ln t A 0 kt •A plot of ln[A]t versus t is a straight line with slope -k and intercept ln[A]0 Second-Order Reactions • A second-order reaction is one whose rate depends on the reactant concentration to the second power or on the concentration of two reactants, each raised to the first power. • For a 2nd order reaction with just one reactant: A 2 Rate k A t 1 1 kt At A0 calculus y = mx + b A plot of 1/[A]t versus t is a straight line with slope k and intercept 1/[A]0. (slope= k) 1/[A]t Time (s) Half-life • Half-life, t½ , is the time required for the concentration of a reactant to decrease to half its original value. • That is, half life, t½, is the time taken for [A]0 to reach ½ [A]0. • Mathematically, the half life of a first-order reaction is: At ln kt A0 So, for t t1 2 and At 1 2 A 0 ln 1 2 A 0 A 0 kt1 2 ln1 2 0.693 t1 2 k k Radioactive decay is 1st order. Note that the half-life of a first-order reaction is independent of the initial concentration of the reactant. • We can show that the half-life of a second order reaction is: 1 t1 2 k A 0 • Note that the half-life of a second-order reaction is dependent on the initial concentration of reactant. Sample Exercise #1 • The first order rate constant for the decomposition of a certain insecticide in water at 12oC is 1.45 yr-1. A quantity of this insecticide is washed into a lake on June 1, leading to a concentration of 5.0 x 10-7 g/cm3 of water. Assume that the effective temperature of the lake is 12oC. (a) What is the concentration of the insecticide on June 1 of the following year? (b) How long for the conc. of the insecticide to drop to 3.0 x 10-7 g/cm3? (a) ln [A]t = - k t + ln [A]0 ln [A] t = - (1.45 yr-1)(1.00 yr) + ln(5.0 x 10-7) eln [A] t = e-15.96 [A] t = 1.2 x 10-7 g/cm3 (note: conc. units for [A] and [A]0 must be the same) (b) ln [A]t = - k t + ln [A]0 ln (3.0 10-7) = - (1.45)(t) + ln (5.0 10-7) t = 0.35 yr • Using the figure to the right, estimate the half-life of the reaction of C4H9Cl with water. • The initial value of [C3H9Cl] is 0.100 M. • The half-life for this reaction is the time for [C3H9Cl] to be 0.050 M. At the end of the second half-life, about 680 s, the should decrease by another factor of 2 • This point occurs at concentration to about 0.025 M. approx. 340 s. The graph indicates that this is true. The reaction SO2Cl2 SO2 + Cl2 is first order in SO2Cl2. Using the following kinetic data, determine the magnitude of the first order rate constant: Time (s) Pressure SO2Cl2 (atm) ln Pressure SO2Cl2 0 1.000 0 2500 0.947 - 0.0545 5000 0.895 - 0.111 7500 0.848 - 0.165 10000 0.803 - 0.219 Graph ln P vs. time (first order) The graph is linear with slope of about - 2.19 10-5 s-1 slope = y (-0.219 - 0) = = - 2.19 x 10 -5 x (10000 - 0) k = - (slope) = 2.19 x 10-5 Sample Exercise #4 The following data was obtained for the gas phase decomposition of NO2(g) at 300oC: 2 NO2(g) 2 NO(g) + O2(g) Time (s) [NO2] (M) 0.0 0.01000 50.0 0.00787 100.0 0.00649 200.0 0.00481 300.0 0.00380 What is the rate law for this reaction? • To test whether the reaction is first or second order, we can construct plots of ln [NO2] and 1 / [NO2] against time. Time (s) 0.0 50.0 100.0 200.0 300.0 [NO2] 0.01000 0.00787 0.00649 0.00481 0.00380 ln [NO2] 1/[NO2] -4.610 -4.845 -5.038 -5.337 -5.573 100 127 154 208 263 From the graphs above, only the plot of 1/[NO2] is a straight line. Thus, the reaction is second order in NO2. Rate = k [NO2]2 From the slope of the straight line graph, we can get: k = 0.543 M-1 s-1 The decomposition of sulfuryl chloride (SO2Cl2) is a first-order process. The rate constant for the decomposition at 660 K is 4.5 10-2 s-1. (a) if we begin with an initial SO2Cl2 pressure of 375 torr, what is the pressure of this substance after 65 s? (b) at what time will the pressure of SO2Cl2 decline to one-tenth its initial value? (a) ln Pt = - kt + ln Po ln P65 = - 4.5 10-2 s-1 (65) + ln(375) P65 = 20 torr (b) Pt = 0.10 P0 = (.10)(375) = 37.5 torr ln Pt = - kt + ln Po ln (37.5) = - (4.5 x 10-2 s-1 )t + ln (375) t = 51 s Consider the data presented (a) By using appropriate graphs, determine whether the reaction is first or second order. (b) What is the value of the rate constant for the reaction? (c) What is the half life for the reaction? 1/[A] (a) make both first and second order plots to see which is linear the plot of 1/[A] vs. time is linear, so the reaction is second order in [A] (b) the slope of the line in the graph is the rate constant slope = y (3.2 - 1.5) = =0.043 M 1min 1 x (40 - 0) (c) use the half-life formula for a 2nd order reaction: t1/2 = 1 / k[A]o = 1 / [(0.043 M-1 min-1) (0.65 M)] = 36 min 14.5 Temperature and Rate • Most reactions speed up as temperature increases • The rate law has no temperature term in it, so the rate constant (k) must depend on temperature. – We see an approximate doubling of the rate with each 10oC increase in temperature. The Collision Model • Rates of reactions are affected by concentration and temperature. • An explanation is provided by the collision model, based on kinetic-molecular theory. • In order for molecules to react they must collide. • The greater the number of collisions the faster the rate. - The more molecules present, the greater the probability of collision faster rate - The higher the temperature, the faster molecules move and more often they collide faster rate However, not all collisions lead to products. • In fact, only a small fraction of collisions lead to products. • In order for a reaction to occur the reactant molecules must collide in the correct orientation AND with enough energy to form products. • The Orientation Factor • The orientation of a molecule during collision can have a profound effect on whether or not a reaction occurs. • Consider the reaction between Cl and NOCl: • If Cl collides with Cl of NOCl, the products are Cl2 and NO. • If the Cl collides with the O of NOCl, no products are formed. Cl Cl-N=O Cl O=N-Cl Activation Energy • Arrhenius: molecules must posses a minimum amount of energy to react. In order to form products, bonds must be broken in the reactants. • Bond breakage requires energy. (ΔHrxn = bonds broken – bonds formed) • Molecules moving too slowly, with too little kinetic energy, don’t react when they collide. • Activation energy, Ea is the minimum energy required to initiate a chemical reaction (varies with the reaction). • Energy is required to stretch and break the bond between the CH3 group and the NC group • The species at the top of the barrier is called the activated complex or transition state. • Energy is released when the C-C bond is formed. • The change in energy for the reaction is the difference in energy between CH3NC and CH3CN. • The activation energy is the difference in energy between reactants, (CH3NC) and the transition state. The rate depends on the magnitude of the Ea. In general, the lower the Ea, the faster the rate. • Notice that if a forward reaction is exothermic (CH3NC CH3CN), then the reverse reaction is endothermic (CH3CN CH3NC). How does this relate to temperature? • At any particular temperature, the molecules present have an average kinetic energy. – Some molecules have less energy than the average while others have more than the average value. • Molecules that have an energy equal to or greater than Ea have sufficient energy to react. • The fraction of molecules with an energy equal to or greater than Ea is given by: f=e -Ea RT (R is 8.314 J/mol-k , Temp in K, Ea in J) At a higher temperature, more molecules have the minimum Ea needed to react and the rate is faster. The Arrhenius Equation • Arrhenius discovered that most reaction-rate data obeyed an equation based on three factors 1. The number of collisions per unit time. 2. The fraction of collisions that occur with the correct orientation. 3. The fraction of the colliding molecules that have an energy equal to or greater than Ea. Arrhenius equation: k=Ae -Ea RT • Where k is the rate constant, Ea is the activation energy(J), R is the ideal-gas constant (8.314 J/K•mol) and T is the temperature (K). • A is the frequency factor. – It is related to the frequency of collisions and the probability that a collision will have a favorable orientation. More Useful Versions of the Arrhenius Equation lnk = - Ea + lnA RT y = mx + b • A graph of ln k vs 1/T will be a line with slope of –Ea/R and a y-intercept of ln A. ln A slope = -Ea / R ln k 1/T k1 Ea 1 1 ln = - k2 R T2 T1 • Useful when we have 2 different k values at 2 different temperatures SAMPLE 1: Consider a series of reactions having the following energy profiles: Assuming that all three reactions have nearly the same frequency factors, rank the reactions from slowest to fastest. Solution The lower the activation energy, the faster the reaction. The value of ∆E does not affect the rate. Hence the order is (2) < (3) < (1). Calculate the fraction of atoms in a sample of argon gas at 400 K that have an activation energy of 10.0 kJ or greater. f = e-Ea /RT Ea = 10.0 kJ/mol = 10000 J/mol 10000 J/mol - Ea /RT = = - 3.01 (8.314 J/mol K)(400 K) f = e-3.01 = 0.049 T = 400 K The gas phase reaction Cl(g) + HBr(g) HCl(g) + Br(g) has an overall enthalphy change of - 66 kJ. The activation energy for the reaction is 7 kJ. (a) Sketch the energy profile for the reaction and label Ea and ΔE. (b) What is the activation energy for the reverse reaction? (b) the reverse reaction has an Ea value of 73 kJ A certain first order reaction has a rate constant of 2.75 10-2 s-1 at 20oC. What is the value of k at 60oC if Ea = 75.5 kJ/mol T1 = 293 K T2 = 333 K k1 Ea 1 1 ln = - k2 R T2 T1 2.75 x102 75.5 x 103 J/mol 1 1 ln = k 8.314 J/mol 333 293 2 2.75 x102 ln = - 3.7 k2 2.75 x102 = 0.024 k2 2.75 x102 s-1 k2 = 1.1 s-1 0.024 The rate of the reaction CH3COOC2H5 + OH-1 CH3COO-1 + C2H5OH was measured at several temperatures and the following data were collected. Using the data, construct a graph of ln k versus 1/T and determine the value of Ea slope = y (-2.955 - -1.103) = = - 5.6 x 103 x (3.47 - 3.14) the slope = -Ea / R Ea = - (-5.6 103)(8.314 J/mol) = 47000 J/mol or 47 kJ/mol The activation energy of a certain reaction is 65.7 kJ/mol. How many times faster will the reaction occur at 50oC rather then 0oC assuming equal initial concentrations? Since we are considering one reaction and temp only affects k which directly affects rate…the ratio of k values equals the ratio of rates at the two temperatures. T1 = 323 K ; T2 = 273 K k 1 65.7 x 103 J/mol 1 1 ln = k 8.314 J/mol 27 3 32 3 2 k1 ln = 4.48 k2 k1 = 88 k2 The reaction will occur about 88 times faster at 50oC 14.6 Reaction Mechanisms • A chemical equation provides information about substances present before and after a reaction. • The reaction mechanism is the process of how the reactants become products. • Mechanisms provide a picture of which bonds are broken and formed during the course of a reaction. • Most reactions occur as a series of small steps or changes. Elementary steps are processes that occur in a single step. • The number of molecules present in an elementary step is the molecularity of that elementary step. • Unimolecular: one molecule in the elementary step. • Bimolecular: two molecules in the elementary step. • Termolecular: three molecules in the elementary step. Multistep Mechanisms • consist of a sequence of elementary steps. – The elementary steps must add together to give the balanced chemical equation. • Intermediates - these are species that appear in an elementary step but are neither a reactant nor product. – They are formed in one elementary step and consumed in another. – They are NOT found in the balanced equation for the overall reaction. Rate Laws for Elementary Steps • The rate laws of the elementary steps determine the overall rate law of the reaction. • For elementary processes, we DO NOT need to find the reaction order by experimentation – it matches the stoichiometry of the equation • The rate law of an elementary step is determined by its molecularity. • Unimolecular processes are first order. • Bimolecular processes are second order. • Termolecular processes are third order. Rate Laws for Multistep Mechanisms • When a reaction occurs by mechanisms with more than one step, the slowest step limits the overall reaction rate. – This is called the rate-determining step of the reaction. – This step governs the overall rate law for the overall reaction. Consider the reaction: NO2(g) + CO(g) → NO(g) + CO2(g) Here is a proposed a mechanism for the reaction: Step 1: NO2(g) + NO2(g) NO3(g) + NO(g) slow step Step 2: NO3(g) + CO(g) NO2(g) + CO2(g) fast step (Note that NO3 is an intermediate.) The overall reaction rate will depend on the slowest step Rate = k[NO2]2 If we do an experiment and find that the experimentally derived rate law is: Rate = k[NO2]2 Then our theoretical rate law is in agreement with the experimental rate law. This supports (but does not prove) our mechanism. The following mechanism has been proposed for the reaction of methane gas with chlorine gas. All species are in the gas phase. Step 1 Cl2 2 Cl fast equilibrium Step 2 CH4 + Cl CH3 + HCl slow Step 3 CH3 + Cl2 CH3Cl + Cl fast Step 4 CH3Cl + Cl CH2Cl2 + H fast Step 5 H + Cl HCl fast (e) Identify the order of the reaction with respect to each of the following according to the mechanism. In each case, justify your answer. (i) CH4(g) (ii) Cl2(g) • The overall rate law depends on the rate law of the slow step: step 2: rate = k[CH4][Cl] *The problem is we can’t use intermediates in overall rate law… Since step 1 is equilibrium the forward and reverse rates are equal. k[Cl2]=k[Cl]2 Rearranging: 𝐶𝑙 = 𝑘 [𝐶𝑙2 ] 𝑘 = 𝑘[𝐶𝑙2 ] 1 2 Plugging back into the rate law for step 2: 1 𝑅𝑎𝑡𝑒 = 𝑘 𝐶𝐻4 𝑘[𝐶𝑙2 ] 2 1 𝑅𝑎𝑡𝑒 = 𝑘 𝐶𝐻4 [𝐶𝑙2 ] 2 14.7 Catalysis • A catalyst is a substance that changes the speed of a chemical reaction without any permanent change to itself – Works by providing an alternate mechanism for the reaction – one that has a lower activation energy • Lowering the activation energy allows for more molecules with Ea large enough to react – more collisions that can make products – higher rxn rate (without changing temperature) – Very common in nature – Much research to find new catalysts AND, much research to find “inhibitors”(substances that slow down an undesired reaction) Example: corrosion • Homogeneous catalyst – catalyst is in the same phase as reactants • Heterogeneous catalyst – catalyst is in different phase than reactants • Enzymes – catalysts in living organisms Effect of a catalyst on a reaction A catalyst provides an alternate mechanism that has a lower Ea than the original mechanism – this allows the reaction to occur at a faster rate What is the molecularity of each of the following elementary processes? Write the rate law for each. (a) Cl2 2 Cl (b) OCl-1 + H2O HOCl + OH-1 (c) NO + Cl2 NOCl2 (a) unimolecular rate = k [Cl2] (b) bimolecular rate = k [OCl-1] [H2O] (c) bimolecular rate = k [NO] [Cl2] (a) (b) (c) (d) Based on the following reaction profile, how many intermediates are formed in the reaction A D? How many transition states are there? Which step is the fastest? Is the reaction A D exothermic or endothermic? This is a three-step mechanism, A B, B C, C D (a) 2 intermediates, B and C (A is reactant, D is product) (b) 3 energy maxima, so there are 3 transition states (c)Step C D has the lowest activation energy, so it is the fastest (d)The energy of D is greater than the energy of A, so the overall reaction is endothermic The following mechanism has been proposed for the gas-phase reaction of H2 with ICl: H2 + ICl HI + HCl HI + ICl I2 + HCl (a) Write the balanced equation for the overall reaction (b) Identify any intermediates (c) Write rate laws for each elementary reaction in the mechanism (d) If the first step is slow and the second one is fast, what rate law do you expect to be observed for the overall reaction? (e) If the second step is the rds, what rate law would you expect for the reaction (a) H2 + ICl HI + HCl HI + ICl I2 + HCl H2 + 2 ICl I2 + 2 HCl (b) HI (c) first step: rate = k1 [H2] [ICl] second step: rate = k2 [HI] [ICl] (d) rate = k [H2] [ICl] (e) rate = k [H2] [ICl]2 The reaction 2 NO + Cl2 2 NOCl obeys the rate law: rate = k [NO]2 [Cl2]. The following mechanism has been proposed for this reaction: NO + Cl2 NOCl2 NOCl2 + NO 2 NOCl (a) What would the rate law be if the first step were rate determining? (b) Based on the observed rate law, what can we conclude about the relative rate of the the two steps? (a) rate = k [NO] [Cl2] (b) Assuming the proposed mechanism is correct the 2nd step is the r-d-s (slower step). (The rate law based on this mechanism with the 2nd step as the r-ds matches the experimentally determined rate law.) The oxidation of SO2 to SO3 is catalyzed by NO2. The reaction proceeds as follows: NO2(g) + SO2(g) NO(g) + SO3(g) 2 NO(g) + O2(g) 2 NO2(g) (a) Show that the two reactions can be summed to give the overall oxidation of SO2 by O2 to give SO3. Hint: the top reaction must be multiplied by a factor so the NO and NO2 cancel out. (b) Why do we consider NO2 a catalyst and not an intermediate in this reaction? (c) Is this an example of homogeneous catalysis or heterogeneous catalysis? (a) 2 [NO2 + SO2 NO + SO3] = 2 NO2 + 2 SO2 2 NO + 2 SO3 2 NO + O2 2 NO2 __ 2 SO2 + O2 2 SO3 (b) NO2 is a catalyst because it is present at the beginning of the reaction – it is consumed and then reproduced in the reaction sequence. (NO is an intermediate because it is produced and then consumed.) (c) homogeneous catalysis