Chemical Kinetics - Fall River Public Schools
advertisement

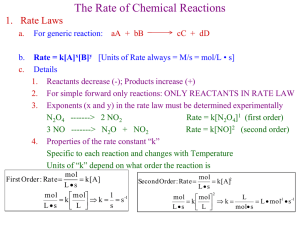
AP Chem Area of chemistry that deals with rates or speeds at which a reaction occurs The rate of these reactions are affected by several factors observable property Rate time Such as: Concentration Color Bubbles Temp pH Whatever is appropriate: Hours Minutes Seconds Candle Wax Example Collisions cause reactions! ◦ Breaking of bonds directly linked to rate ◦ Must overcome repulsion of electron clouds Also correct orientation sometimes needed ◦ Example: Chalk dropping Concentration of reactants ◦ With gases, pressure used instead Temperature at which the reaction occurs The presence of a catalyst Surface area of solid or liquid reactants Demo: ◦ Chalk + 1 M HCl / Chalk + 1 M Acetic Acid Prediction? Factor? ◦ Chalk + 1 M HCl / Chalk + 6 M HCl Prediction? Factor? ◦ Chalk + 1 M HCl (Room Temp) / Chalk + 1 M HCl (Heated) Prediction? Factor? Speed of reaction or reaction rate is the time over which a change occurs Consider the reaction A B Reaction rate is a measure of how quickly A is consumed or B is produced Average rate of reaction can be written: (moles B) Average rate t This is a measure of the average rate of appearance of B Average rate can also be written in terms of A: (moles of A) Average rate t This is the rate of disappearance of A (equal to B only negative) Average Rates can only be positive Start with one mole of A at time zero, measure amounts of A and B at given time intervals Data for Reaction A B When mole ratios of equations are not 1:1 For the reaction: aA + bB cC + dD Example ◦ If the rate of decomposition of N2O5 in a reaction vessel is 4.2 x 10-7 M/s, what is the rate of appearance of NO2 and O2 2 N2O5(g) 4 NO2(g) + O2(g) Consider the reaction between butyl chloride and water: C4H9Cl(aq) + H2O(l) C4H9OH(aq) +HCl(aq) C4 H 9Cl Average rate t Using the curve created from the data, we can determine the instantaneous rate for any given point on the curve Recall: slope is rise over run! Analogy: Distance between Fall River and Norton is 29.7 mi along a certain route. It takes Mr. N 30 minutes to get to school. His average rate is 59.4 mph. But at t = 15, Mr. N’s instantaneous rate is 95 mph, and at t = 1 Mr. N’s instantaneous rate is 25 mph. Increasing concentration of reactants gives increasing rate Decreasing rates of reactions over time is typical ◦ Due to decreasing concentration of reactants Rates of a reaction can be related to concentrations with a rate constant (k) For reaction: aA + bB cC + dD Rate kA B x y Rate laws are defined by reactant (not product) concentrations For the rate law expression: Rate kA B x y The overall order of reaction is the sum of the powers (x + y) ◦ However, rate with respect to [A] is only x In most rate laws reaction orders are 0, 1, or 2 ◦ Can be fractional or negative at times ◦ Most commonly 1 or 2 Reaction orders are determined experimentally, and do not necessarily relate to coefficients of a balanced equation Example: What is the overall order of reaction for the reaction below? CHCl3(g) + Cl2(g) CCl4(g) + HCl(g) Rate= k [CHCl3][Cl2]1/2 A.) ½ B.) 2 C.) 3/2 D.) 2/2 Zero order for a reactant means concentration changes have no effect on reaction rate ◦ Example: Drinking 1st order means concentration changes give proportional changes in reaction rate ◦ Double the concentration, double the rate 2nd order rate law, increasing in concentration results in a rate increase equal to the concentration increased to the second power ◦ Example: Double conc. = 22 = 4 (rate increase) Triple conc. = 32 = 9 (rate increase) The units for the rate constant depend on the order of the rate law units of rate Unitsof rateconstant (unitsof concentrat ion)Z M /s units of rate constant Z M Z = overall order of reaction Rate Law Overall Order Units of Rate Constant (k) Rate = k Zero M/s (M s-1) Rate = k [A] First 1/s (s-1) Rate = k [A][B] Second 1/M s (M-1 s-1) Rate = k [A]2[B] Third 1/M2 s (M-2 s-1) What is the unit for the rate constant for the reaction below? CHCl3(g) + Cl2(g) CCl4(g) + HCl(g) Rate= k [CHCl3][Cl2]1/2 A.) M½/s B.) M/s C.) M2/s D.) M-1/2/s A particular reaction was found to depend on the concentration of the hydrogen ion. The initial rates varied as a function of [H+] as follows: [H+] (M) 0.0500 0.100 0.200 Initial rate(M/s) 6.4x10-7 3.2x10-7 1.6x10-7 What is the order of the reaction in [H+] A.) 1 B.) 2 C.) -1 D.) -1/2 Rate Data for the Reaction Between F2 and ClO2 [F2] (M) [ClO2] (M) Initial Rate (M/s) 0.10 0.010 1.2 x 10-3 0.10 0.040 4.8 x 10-3 0.20 0.010 2.4 x 10-3 What is the rate law expression for the reaction? Rate law tells how rate changes with changing concentrations at a particular temperature We can derive equations that can give us the concentrations of reactants or products at any time during a reaction (instantaneous) ◦ These are known as integrated rate laws Rate = k Using calculus, the integrated rate law is: [ A]t kt A0 [A]t is concentration of reactant at time t [A]0 is initial concentration of reactant [ A]t kt A0 y m x b This has the same form as the general equation for a straight line Graphically, the slope is equal to -k Separate equations can be derived relating to time required for reactants to decrease to half of initial concentration (aka half-life or t1/2) When t = t1/2, [A]t is half of [A]0 ([A]t =[A]0/2) Rate = k [A] Using calculus, the integrated rate law becomes: lnAt k t lnA0 This equation is of the general equation for a straight line (like before) Note that only the second graph is used so that the slope can be determined Also, y-intercept is ln [A]0 Example 2N2O5(g) 4NO2(g) + O2(g) The decomposition of dinitrogen pentoxide is a 1st order reaction with a rate constant of 5.1 x 10-4 s-1 at 45ºC. If initial conc. is 0.25M, what is the concentration after 3.2 min.? Example #2 2N2O5(g) 4NO2(g) + O2(g) The decomposition of dinitrogen pentoxide is a 1st order reaction with a rate constant of 5.1 x 10-4 s-1 at 45ºC. How long will it take for the concentration of N2O5 to decrease from 0.25M to 0.15M? For first order reactions: t1/ 2 0.693 k Note it is independent of concentration! This is used to describe radioactive decay and elimination of medications from the body Rate = k [A]2 Using calculus, the integrated rate law becomes: 1 1 kt [ A] [ A]0 Just like the previous two, this equation is of the general equation for a straight line Unlike first order, second order does depend on initial concentrations: 1 k[ A]0 Ways to find Rate Constant or Reaction Order Conc. Vs. Time (Graphically) k = slope Rates vs. Conc. (Exp. Data/Rate Laws) Half-life expressions Most reactions increase in rate with increasing temperature This is due to an increase in the rate constant with increasing temperature Minimum amount of energy required to initiate a chemical reaction ◦ Varies from reaction to reaction This is the kinetic energy required by colliding molecules in order to begin a reaction ◦ Remember, even with sufficient KE, orientation is still important Activation energy must be enough to overcome initial resistance for a reaction to take place Diagram can be used to determine if reaction is exothermic (- ∆H) or endothermic (+∆H) Activated complex (or transition state) is the arrangement of atoms at the peak of the Ea barrier ◦ Unstable and only appears briefly The relationship between rate and temperature was non-linear Reaction rate obeyed an equation based on 3 factors: 1. Fraction of molecules that possess Ea 2. # of collisions per second 3. Fraction of collisions with proper orientation k Ae Ea / RT k = the rate constant R = gas constant (8.314 J/mol*K) T = Absolute temperature (K) Ea = the activation energy A = frequency factor ◦ A is mostly constant with variations in temperature Taking the natural log of both sides gives a formula in straight line form: Ea ln k ln A RT Graph of ln k versus 1/T will be a straight line with a slope of –Ea/R and a y-intercept of ln A In order to compare different rates at different temperatures the equation can be rearranged: k1 Ea 1 1 ln k2 R T2 T1 Example ◦ The rate constant of a first order reaction is 3.46 x 10-2 s-1 at 298 K. What is the rate constant at 350 K if the activation energy is 50.2 kJ/mol? k1 = 3.46 x 10-2 s-1 T1= 298 K k2 = ? T2 = 350 K Example -2 1 -2 1 3.46x 10 s 50.2kJ K mol 1 1 ln k2 mol 8.314J 350K 298K 3.46x 10 s ln k2 50200J K mol 4 4.98 x10 mol 8.314J -2 3.46x 10 s ln k2 1 3.01 -2 3.46x 10 s k2 -2 1 3.46x 10 s .0493 .0493 1 k2 = 0.702 k2 -1 s The process by which a reaction is broken down into multiple step reactions ◦ Chemical equations only show beginning and ending substances ◦ Can show in detail bond breaking and forming and structural changes that occur during a reaction Elementary steps ◦ Processes that occur in a single event or step, are elementary processes. Can determine rate laws from elementary steps, unlike overall reactions ◦ Particles collide with sufficient energy and proper orientation for reaction to occur ◦ These are the small step reactions in which an overall reaction occurs Often times chemical reactions are a result of multiple steps not shown by the overall equation NO2( g ) CO( g ) NO( g ) CO2( g ) The above rxn below 225 °C occurs as 2 elementary steps 1st step ◦ 2 NO2 molecules collide NO NO2( g ) NO3( g ) NO( g ) nd 2( g ) 2 step ◦ The NO3 then collides with CO and transfers an O NO CO NO CO 3(g) (g) 2(g) The elementary steps must add to result2(g) in the overall chemical equation Every reaction is made up of a series of elementary steps Rate laws reflect the relative speeds of these steps The rate law of an elementary step is directly related to its molecularity ◦ This is the number of molecules that participate as reactants Unimolecular (Aproduct) ◦ Rate =k[A] Bimolecular (A+B prod) = 1st order = 2nd order ◦ Rate= k[A][B] Most reactions involve multiple steps Often one step is much slower than the other The Rate Determining Step (RDS) is the slowest step in the reaction The slowest step of a multi-step reaction determines the overall rate of reaction! Good things to know/determine: ◦ Steps must add up to overall reaction ◦ Identify Catalyst Consumed at first, regenerated later (not in overall) ◦ Identify Intermediates Produced and then consumed later ◦ Each step has a rate law Depends on number of reactants ◦ Rate Determining Step => slowest step Example: ◦ What is the rate law of the following multi-step reaction? step1: NO2( g ) NO2( g ) NO3( g ) NO( g ) slow k1 step2 : NO3(g) CO(g) NO2(g) CO2(g) k2 fast Overall: NO2( g ) CO( g ) NO( g ) CO2( g ) As a general rule catalysts change the rate of reaction by lowering the Ea Usually this is done by giving completely different mechanism for the reaction ◦ This lowers the overall Ea