Vibrational Spectroscopy
advertisement

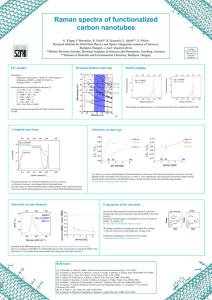
Spectroscopy of Hybrid Inorganic/Organic Interfaces Vibrational Spectroscopy Dietrich RT Zahn The Application of Raman Spectroscopy in the DIODE Project The Overall Device Performance (iv) The Interface between the Organic Molecules and the Metal Metal I V (iii) The Organic Molecular Film Organic Interlayer GaAs(100) (ii) The Interface between GaAs Substrate and Organic Molecules (i) The GaAs Substrate Surface Dietrich RT Zahn, TU Chemnitz Molecular Vibrational Properties PTCDA: 3,4,9,10- Perylenetetracarboxylic diAnhydride DiMe-PTCDI: 3,4,9,10- Perylenetetracarboxylic diImide C24H8O6 Symmetry D2h Raman active: 19Ag+18B1g+10B2g+7B3g IR active: +10B1u+18B2u+18B3u Silent: + 8Au 108 internal vibrations C26H14O4N2 C2h 44Ag+22Bg +23Au+43Bu + 8Au 132 internal vibrations Monoclinic crystallographic system in thin films: • PTCDA: - and -phases: S. R. Forrest, Chem. Rev. 97 (1997), 1793. • DiMe-PTCDI: Cambridge Structural Database. Dietrich RT Zahn, TU Chemnitz Raman-active vibrations of PTCDA (C24H8O6): Effect of crystal formation internal molecular modes: Symmetry: D2h Davydov Splitting C- C- O Bg C-C Bg 200 300 400 500 600 700 C-H x2 C-C 1200 1300 1400 1500 1600 1700 -1 Raman shift / cm 6 rotational vibrations: 3Ag+3Bg Ag Intensity / a.u. 2-fold Intensity / a. u. 19Ag+18B1g +10B2g+7B3g external molecular modes (phonons): C2h (monoclinic) Ag Bg Ag 25 50 75 100 125 -1 Raman shift / cm Dietrich RT Zahn, TU Chemnitz Vibration modes of PTCDA molecule B3LYP / 3-21G DFTB Mode freq / cm-1 freq / cm-1 freq / cm-1 12 19 25 29 33 41 49 64 67 75 76 79 83 86 92 94 100 103 107 233 389 476 537 624 727 858 1054 1150 1305 1340 1381 1389 1544 1572 1590 1774 233 383 474 550 639 728 863 1070 1140 1285 1304 1347 1393 1527 1616 1623 1723 3173 3190 233 389 476 539 627 732 853 1059 1151 1269 1303 1346 1389 1472 1611 1620 1800 3227 3253 Raman Activity 0,99 6,43 0,88 122,86 72,23 12,01 2,63 346,71 161,30 1030,13 0,00 5362,37 551,12 1083,97 0,01 1395,17 1284,66 173,49 236,78 Intensity / a. u. Exp C-C C-O B g 200 300 C-H 400 500 600 700 x2 C-C 1200 1300 1400 1500 1600 1700 -1 Raman shift / cm 19 Ag breathing modes very good agreement between experimental and calculated frequencies ! Dietrich RT Zahn, TU Chemnitz Intensity / arb. units Raman Spectra of a PTCDA Crystal x0.1 200 400 600 1200 1350 Raman shift / cm 1500 1650 -1 • assignment of modes and their relative atomic contribution using Gaussian `98 (B3LYP, 3-21G) Dietrich RT Zahn, TU Chemnitz Intensity / arb. units Ag Raman Modes of PTCDA with In x0.1 200 400 600 1200 1350 Raman shift / cm 1500 1650 -1 Dietrich RT Zahn, TU Chemnitz Raman Spectra of a PTCDA Crystal and a DiMe-PTCDI Intensity / arb. units C-H ring C=O x0.5 x0.1 200 400 600 1200 1350 Raman shift / cm 1500 1650 -1 • assignment of modes and their relative atomic contribution using Gaussian `98 (B3LYP:3-21G). Dietrich RT Zahn, TU Chemnitz Intensity / arb. units Raman Spectra of a PTCDA Crystal and a DiMe-PTCDI ωDiMe-PTCDI mPTCDA = =0.97 ωPTCDA mDiMe-PTCDI 200 400 600 1200 1350 ωDiMe-PTCDI 1500 221 = 1650 =0.95 ω 233 PTCDA -1 experimental Raman shiftRaman /cm-1 shift / cm • assignment of modes and their relative atomic contribution using Gaussian `98 (B3LYP:3-21G). Dietrich RT Zahn, TU Chemnitz Raman-active vibrations of PTCDA: Effect of crystal formation external molecular modes (phonons): 6 rotational vibrations: 3Ag+3Bg Symmetry: C2h (monoclinic) Bg Intensity / a.u. Ag Bg 25 50 75 100 125 -1 Raman shift / cm Dietrich RT Zahn, TU Chemnitz Infrared Modes in Films on S-GaAs Reflection, s-polarized light. C=O Rsample/Rsubstrate 1.4 C-O+ C-C 1.3 1.2 ring C-H+ C-N-C C-O-C C-H 1.1 (oop) 1.0 750 1000 1250 1500 -1 1750 Wavenumber / cm •Assignment of modes using Gaussian `98 (B3LYP, 3-21G). Dietrich RT Zahn, TU Chemnitz Epi-ready GaAs (100) Sample Preparation Degreasing Acetone, Ethanol, Di-Water Wet Chemical Treatment S2Cl2:CCl4=1:3 (10 sec) OMBD deposition: PTCDA, DiMe-PTCDI Thickness: 0.1 nm ÷15 nm Rinsing (CCl4, Acetone, Ethanol, Di-Water) Annealing at 620 K, 30 min Metal deposition: Ag, In Thickness: 0.1 nm ÷260 nm S-GaAs(100):2x1 Dietrich RT Zahn, TU Chemnitz Ex Situ (Micro-) and In Situ (Macro- Configuration) Raman Spectroscopy Absorbtion coefficient *10 5 Wavelength / nm Dilor XY 800 Spectrometer 800 700 600 6 500 S0-S1 transition 400 Ar+ line 4 4 S0-S2 transition 2 2 DiMe-PTCDI 0 PTCDA 0 1.5 2.0 2.5 3.0 3.5 Energy / eV Monochromatic light source: Ar+ Laser (2.54eV), Detector: CCD • resonance condition with the absorption band of the organic crystalline material. • resolution: 1.2 cm-1 to 3.5 cm-1. Dietrich RT Zahn, TU Chemnitz 4.0 E = 2.54 eV Normalized Intensity Monitoring of PTCDA Film Growth on S-GaAs 1.0 0.8 0.6 -1 431 cm -1 386 cm -1 233 cm simulation, k= 0.99 0.4 0.2 0.0 0 20 40 60 80 100 120 Thickness / nm M. Ramsteiner et al., Appl. Opt. 28 (18) (1989), 4017. • The relative intensity of internal modes does not change upon deposition. weak interaction of the molecules with the S-passivated substrate. • Phonons are well resolved as soon as 20 nm of PTCDA are deposited. Dietrich RT Zahn, TU Chemnitz 40 nm x 0.01 0.002 -1 Intensity / cts mW s -1 Chemistry at Organic/S-GaAs(100):2x1 Vibrational Properties: PTCDA Annealing at 623 K for 30 0.45 nm (x 0.6) 0.18 nm ann. x 4.4 1300 1400 1500 -1 Raman shift / cm 1600 min: • Molecules remaining at the surface: NPTCDA(0.04nm)~1013 cm-2 NdSi ~ 1012 cm-2 • Spectrum of annealed film similar to that of an annealed PTCDA film on Si(100). The strongest interaction: between the PTCDA molecules and defects due to Si at the GaAs surface. Dietrich RT Zahn, TU Chemnitz Calculated Vibrational Properties: PTCDA 30 2.7 cm -1 20 1000 4 Intensity / A amu -1 1500 1340 1350 10 500 0 0 300 600 900 1200 1400 1600 -1 Raman shift / cm Dietrich RT Zahn, TU Chemnitz Calculated Vibrational Properties: PTCDA 1500 1000 • significant spectral changes predicted for the C=C modes around 1600 cm-1 4 Intensity /A amu -1 Molecular charging with one elementary charge: 500 negative positive 0 fractional charge transfer between the PTCDA and the defects at the GaAs surface. neutral 1300 1400 1500 1600 1700 Raman shift / cm -1 Dietrich RT Zahn, TU Chemnitz In Situ Raman: Monitoring of Indium Deposition onto PTCDA (15 nm) 0.05 Intensity / cts mW s -1 -1 0.005 In thickness / nm /5 43 26.0 /10 /58 15.0 /33 8.0 /28 5.0 /13 2.8 /1.5 1.1 /0.7 200 400 600 1200 1400 Raman shift / cm -1 0.4 0 1600 Dietrich RT Zahn, TU Chemnitz Influence of Indium on Vibrational Spectra of PTCDA 0.0025 -1 -1 Ag Ag Intensity /cts mW s B3g Ag B B Ag 2u B1u B3g Ag (B3g) B3g B1u 0.0025 Ag Ag 2g B2u Ag In 15 nm GaAs B3g B3g + In PTCDA 200 400 600 1200 Raman shift / cm -1 1400 1600 Dietrich RT Zahn, TU Chemnitz Influence of Indium on Vibrational Spectra of PTCDA R/Rsubstrate 1.6 C-H(z) C-H, C-O 1.4 • organic films grown on S-GaAs(100):2x1 In(15nm)/ PTCDA(15 nm) C-H C-H C-C, C=C • reflection measurements at 20° incidence. C=O C-O 1.0 PTCDA(130 nm) 800 1000 1200 1400 1600 Wavenumber/ cm 1800 2000 -1 • all PTCDA modes are preserved in the spectrum of In/PTCDA. • observation of C=O modes (around 1730-1770cm-1) In does not react with the O of PTCDA ! Dietrich RT Zahn, TU Chemnitz Indium/PTCDA: Separation of Chemical and Structural Properties In: 1 nm/min PTCDA S-GaAs(100) PTCDA (15 nm) In: 0 100 nm PTCDA Intensity / cts mW s -1 -1 ~0.4 nm (~1 ML) PTCDA (0.4 nm) 0.03 0.001 x 0.017 x0.045 ~15 nm (~50ML) + In PTCDA S-GaAs(100) 1200 1350 1500 1650 1350 Raman shift / cm 1500 1650 -1 Dietrich RT Zahn, TU Chemnitz Comparison of Indium and Silver Deposition on PTCDA and DiMe-PTCDI In: 1 nm/min Ag:1.6 ÷ 5.5 nm/min -1 -1 Intensity / cts mW s S-GaAs(100) DiMe-PTCDI (15 nm) PTCDA (15 nm) 1200 0.03 0.01 S-GaAs(100) Ag + In 1400 1600 1200 Raman shift / cm 1400 -1 1600 Dietrich RT Zahn, TU Chemnitz Comparison of Indium and Silver Deposition on PTCDA and DiMe-PTCDI PTCDA 0.4 nm In DiMe-PTCDI 0.4 nm In Intensity /a.u. x4 0.3 nm Ag /2 0.4 nm Ag /2 100 200 100 • the PTCDA external modes: are preserved broadened after 0.3 nm Ag deposition. disappear after 0.4 nm In. • the DiMe-PTCDI external modes: less affected compared to PTCDA. probably due to less compact crystalline structure. 200 -1 Raman shift / cm Dietrich RT Zahn, TU Chemnitz Mg,PTCDA In, Ag on PTCDA (15 nm) 0.03 Intensity / cts.mW s -1 -1 0.01 /5 /10 + Mg + In + Ag PTCDA 200 300 400 500 600 1300 1400 1500 1600 -1 Raman shift / cm Dietrich RT Zahn, TU Chemnitz Mg, In, DiMe-PTCDI Ag on DiMe-PTCDI (15 nm) 5x10 -3 -2 5x10 cts mW -1 -1 cts.mW s -1 -1 s Intensity ++Mg In + In + Ag DiMe-PTCDI 200 300 400 500 600 1300 Raman shift / cm -1 1400 1500 1600 Dietrich RT Zahn, TU Chemnitz Indium and Silver Deposition: Enhancement Factors PTCDA (15 mn) DiMe-PTCDI (15 nm) Ag Thickness / nm Ag Thickness / nm 10 20 30 0 40 100 100 10 10 Relative Area Relative Area 0 -1 1244 cm 1 -1 1570 cm -1 1615 cm 1000 100 20 30 40 -1 1236 cm 1 -1 1568 cm -1 1610 cm 100 10 10 1 10 1 0 10 20 30 40 In thickness / nm 0 10 20 30 40 In thickness / nm Dietrich RT Zahn, TU Chemnitz Determination of Molecular Orientation: =0°: x II [011]GaAs DiMe-PTCDI =90°: x II [0-11] phonons phonons Azimuthal rotation of a 120 nm thick film; normal incidence. Periodic variation of signal in crossed and parallel polarization. M. Friedrich, G. Salvan, D. Zahn et al., J. Phys. Cond. Mater. submitted. Dietrich RT Zahn, TU Chemnitz Determination of Molecular Orientation: DiMe-PTCDI Breathing mode at 221 cm-1 Depolarization Ratio/ a.u. 2.5 2.0 1.5 1.0 0.5 0.0 0 60 120 180 240 300 360 Experimental angle ()/° I = es Ag ei Dep = Ag = R , , , Agm R -1 , , , Iyx I xx 56 4; , Good agreement with IR and NEXAFS results Dietrich RT Zahn, TU Chemnitz Molecular Orientation with respect to GaAs substrate: PTCDA: ~ 9° Dietrich RT Zahn, TU Chemnitz [-110] DiMe-PTCDI: ~ 6° ~ 60° Dietrich RT Zahn, TU Chemnitz Raman Characterization of Organic Thin Films: Achievements and Outlook Interface reactions Film thickness Growth Mode Crystallinity Crystalline Order Crystal modifications Orientation Internal Modes: Shifts, Intensities Intensity modulations Intensity modulations Occurrence of Phonon-like Modes, FWHM Phonons, Davydov Splitting of Internal Modes Further investigations Dietrich RT Zahn, TU Chemnitz Raman Spectroscopy Team: Dietrich RT Zahn, TU Chemnitz