PRESENTATION NAME
advertisement

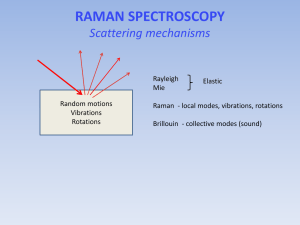
Light 10th Grade - Physics Module Objectives • • • • • Refraction through a prism Dispersion Recombination Uses of spectroscope Raman effect Light - Recap • • • • • • Light is a form of energy Travels in straight lines Refraction is due to change in velocity Refractive index of a medium = velocity of light in vacuum : velocity of light in medium Composite light splits into its constituent colors, when passed through a prism Rainbow is a spectrum formed in nature Difference in refractive index Causes illusional bend in straight object Different medium has different refractive index and hence change in angle of bend • . Dispersion • 1. Seperation or splitting up of composite light into its constituent colours is called . dispersion. 2. Medium that seperates light to its constituent colours is called dispersive medium. 3. Sunlight is made up of various colours. Can be splitted to the same sequence of colours as that of rainbow when passed through prism 4. Dispersion is found by Newton 5. Vacuum/Air is not a dispersive medium Spectrum • • • Band of colours/wavelength, obtained by dispersion of . a narrow beam of composite light is called Spectrum VIBGYOR – Violet, Indigo, Blue, Green, Yellow, Orange, Red Spectrum experiments by Newton Colour is not manufactured by prism. Sunlight consists different colours • . Newton test confirmed that by placing two prisms in inverted positions, found that colours of spectrum produced by Prism 1 recombined in Prism 2 thus emerging as White light. http://www.youtube.com/watch?v=6_HroTxaZe0 Very difficult to seperate colours as colour coming out of small hole is diverging Visible spectrum • • • 400 nm (violet) to 750 nm (Red) Wavelength below the wavelength of violet is called ultraviolet Wavelength above the wavelength of red is called infrared Pure/Impure Spectrum • • Can you find out distinctive yellow colour in the below spectrum? Can you find out distinctive blue colour in the below spectrum? • If we project any spectrum produced by a prism on a screen, colours are always overlapping Spectrums are impure – colours are not distinct • • • Pure Spectrum – Spectrum in which the constituent colours occupy thier respective positions and are distinct How to obtain a pure spectrum ??? Spectroscope • • • • • Instrument to obtain pure spectrum of a polychromatic light using a prism. Lenses are placed before and after the prism to minimise overlapping of colours. Light from the slit is rendered parallel by the collimeter. These rays pass through the prism, get dispersed and enter the telescope. Telescope helps to observe a magnified image of a spectrum. Spectroscope - Terminologies • • • Spectrometer – a circular scale is provided to find position of the telescope. Spectrometer is used to measure angle of prism, refractive index and dispersion. Spectrograph – a camera used in place of the eye-piece to have a permanent record. Direct vision spectroscope – the spectrum of light from a source is seen in the direction of the source. It is a pocket sized instrument meant for quick observation of spectra. • • • Different types of Spectra Continuous emission spectrum • Sunlight, light from a filament lamp, molten iron or a candle flame all give rainbow like spectrum Line emission spectrum • Occurs when different gases or vapours are made to emit light. The spectrum consists of a number of sharp, bright coloured lines against a dark background. Lines are slit images of different colours. • Patterns of lines is different for different elements. Once patterns are known, the elements present in any source can be identified. • This process helps to find the elements present in the sun and other stars. Also, it is possible to find approx. amount of each substance present, by measuring intensities of the lines. • This technique is called spectrochemical analysis and is being used in industries, medical crime detection etc Absorption spectrum • When composite light passes through a semi-transparent substance, some particular colours of the incident radiation are absorbed. Hence the transmitted light lacks particular colours. The corresponding spectrum has a number of dark lines or bands, against the background of continuous spectrum. Different types of Spectra Contd… •Fraunhofer lines •When white light from a carbon arc lamp is passed through sodium vapour, then the continuous spectrum will have two dark lines in the yellow region. The solar spectrum has several dark lines in its spectrum. These are due to the absorption of certain colours by the elements in the solar atmosphere. •The study of these Fraunhofer lines has enabled us to identify the elements present in the solar atmosphere • Fraunhofer lines Tyndall and Rayleigh • • • When a beam of light was allowed to pass through a homogeneous and transparent medium, a portion of the incident light got deflected sideways or scattered. Wavelength of the scattered beam is found to be the same as that of the incident light. Lord Rayleigh – 1871 – Showed that the intensity of scattered light in any medium is inversely proportional to the fourth power of its wavelength. This type of scattering is known as Rayleigh/coherent scattering – no change in wavelength of light Based on Tyndall and Rayleigh • • • • • Sunset and sunrise are red and the sky is blue. Phenomena due to scattering of light by the molecules of the atmosphere. Intensity of scattered light increases rapidly as the wavelength decreases. When sunlight passes through atmosphere, blue colour is scattered most. So sky appears blue. At sunset and sunrise, sunlight passes through maximum length of atmosphere. Much of the blue is taken away by scattering. The light that reaches earth’s surface lacks in blue colour. Hence sunset and sunrise appears reddish. Raman Effect • • • • • C.V. Raman – 1928 – Studied the scattering of light by liquids with the intention of reproducing blue colour of the sea and fo the sky. A beam of monochromatic light was passed through organic liquids such as benzene, toluene etc, the scattered light was no longer monochromatic. Scattered light contained higher and lighter frequencies in addition to that of incident light. This phenomenon is called Raman effect. Raman was awarded Nobel prize in 1930. February 28th is observed as National Science Day, every year in our country to commemorate Raman’s discovery. Raman effect is predicted theoritically by Smekal as early as 1923. Raman is first to observe it experimentally. So sometime, Raman effect is also called as Smekal – Raman effect. Raman Effect • • • • • Special spectroscopes are required to obseve Raman effect. In Raman effect, there are additional frequencies apart from that of incident light. For this reason, Raman scattering is called incoherent scattering. Raman gave satisfactory explanation of incoherent scattering on the basis of quantum theory of radiation. Raman effect has been observed and studied in great number of liquids, vapours, gases and some solids. Raman effect is very useful to understand the structure of molecules that constitute matter. Exercises • Explain Light Refraction based on the below examples. Exercises • • • • • • • • • • • • What is dispersion? How is it caused? Mention the range of wavelengths of visible spectrum. What is meant by a pure spectrum? Explain. What is a spectroscope? Mention the parts of a spectroscope. List three uses of spectroscope. What is a continuous spectrum? What is a line emission spectrum? What are Fraunhofer lines? Explain Raman effect. What is the important difference between Rayleigh scattering and Raman scattering? Mention one application of Raman effect.