Purpose of this lecture

Liquid Phase Properties from VLE Data SVNA 12.1
Purpose of this lecture:
To illustrate how activity coefficients can be calculated from experimental
VLE data obtained at low pressures
Highlights
• For our calculations we take advantage of the fact that as P->0 the vapour phase molecular interactions in a mixture at VLE become very weak, hence the vapour behaves as an ideal gas. In thermodynamic
ˆ
1 .
0
• The modified form of Raoult’s law can then be used for the estimation of the activity coefficients from experimental low P VLE
Reading assignment: Section 12.1 (pp. 430-432)
CHEE 311 Lecture 15 1
7. Liquid Phase Properties from VLE Data SVNA 12.1
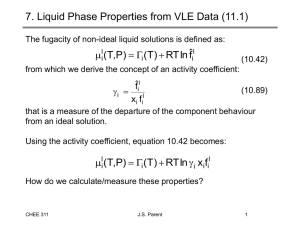
The mixture fugacity of a component in non-ideal liquid solution is defined by:
l i
( T , P )
i
( T )
RT ln fˆ i l
(11.46)
We also define the activity coefficient:
i
x i fˆ i l f i l
(11.91) which is a measure of the departure of the component behaviour from an ideal solution.
Using the activity coefficient, equation 11.46 becomes:
l i
( T , P )
i
( T )
RT ln
i x i f i l
How do we calculate/measure these properties?
CHEE 311 Lecture 15 2
Liquid Phase Properties from VLE Data
Suppose we conduct VLE experiments on our system of interest.
At a given temperature, we vary the system pressure by changing the cell volume.
Wait until equilibrium is established (usually hours)
Measure the compositions of the liquid and vapour
CHEE 311 Lecture 15 3
Liquid Solution Fugacity from VLE Data
Our understanding of molecular dynamics does not permit us to predict non-ideal solution fugacities, f i l . We must measure them by experiment, often by studies of vapour-liquid equilibria.
Suppose we need liquid solution fugacity data for a binary mixture of A+B at P,T. At equilibrium, fˆ i l fˆ i v
The vapour mixture fugacity for component i is given by, fˆ i v i v y i
P
(11.52)
If we conduct VLE experiments at low pressure, but at the required temperature, we can use fˆ i v y i
P by assuming that
i v = 1.
CHEE 311 Lecture 15 4
Liquid Solution Fugacity from Low P VLE Data
Since our experimental measurements are taken at equilibrium, fˆ i l fˆ i v
y i
P
What we need is VLE data at various pressures (all relatively low)
Table 12.1
CHEE 311 Lecture 15 5
Activity Coefficients from Low P VLE Data
With a knowledge of the liquid solution fugacity, we can derive activity coefficients.
i fˆ i l
Actual fugacity
x i f i l
Ideal solution fugacity
Our low pressure vapour fugacity simplifies f i l to give:
i
y i
P x i f i l and if P is close to P i sat : f i l i sat
P i sat exp
V i l
( P
RT
P i sat
)
P i sat leaving us with
i
x i y i
P
P i sat
CHEE 311 Lecture 15 6
Activity Coefficients from Low P VLE Data
Our low pressure VLE data can now be processed to yield experimental activity coefficient data:
i
x i y i
P
P i sat
Table 12.2
CHEE 311 Lecture 15 7
Activity Coefficients from Low P VLE Data
CHEE 311 Lecture 15 8