File - Pace Ap Environmental Science
advertisement

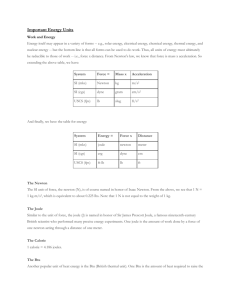
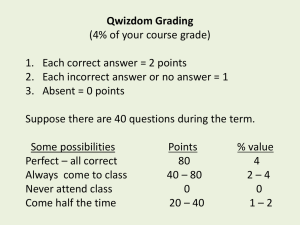
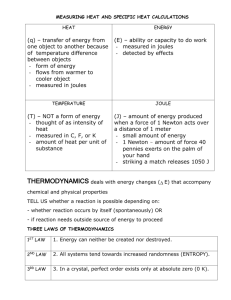
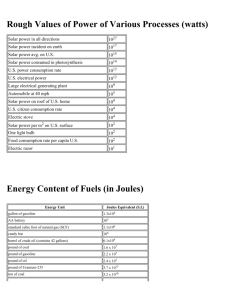
LAWS OF THERMODYNAMICS 1st Law – Conservation of Energy 2nd Law – As energy is converted into another form, some of it is lost to heat. There are no 100% efficient machines; Entropy is always increasing; Perpetual motion machines are impossible. Energy tends to go from more usable (higher quality) to less usable (lower quality). When you use energy, you lower its quality. 3rd Law – Entropy at absolute zero is exactly equal to zero. Which Law of Thermodynamics? a) The amount of energy in an isolated system stays constant. b) An energy transformation occurs and results in increased disorder. WORK & ENERGY Energy = “ability to do work” but what is “work”? Work = force x distance that object moves Thus, we get the idea that energy allows one to move objects from one place to another, thereby accomplishing some physical labor or “work.” Energy can be in many forms (solar, electrical, chemical, thermal, nuclear) but ALL FORMS CAN BE USED TO DO WORK. System Length Mass Time SI Meter Kilogram second USCS foot slug second System Force = Mass x Acceleration SI newton kg m/s2 USCS lb Slug ft/s2 System Energy = Force x Distance SI joule = newton meter USCS ft-lb = lb ft newton (N) Unit of force; 1 N = 1 kg-ms2 joule (J) Unit of energy. The joule is a relatively small amount of energy; used a lot in science. The energy content of a large donut is about 100,000 joules. calorie (c) 1 calorie = 4.186 joules 1 calorie is the heat required to raise the temp. of one gram of water by one Celsius degree. The kilocalorie is known as the “BIG” calorie and written C or Calorie. Food calories are always big Calories. Thus, when one speaks of 100 Calories in a slice of bread, it is actually means that 100 kilocalories or 4.186 x 105 J would be released through burning the dried biomass. Btu (British thermal unit) – amount of heat required to raise the temp. of one pound of water by one degree Fahrenheit. 1 Btu = 252 cal = 1055 One Btu is approx. the amount of heat released by burning one match. Btus are common in water heaters, furnaces, & air conditioners. Water heater could be rated at 40,000 Btu/h Btu/h gives the rate at which heat can be produced. Heating values for fuels are often stated in terms of Btus per unit weight. Ex) Coal – has typical value of 25 million Btu/ton Ex) Petroleum – 37 million Btu/ton Therm - Gas companies in the U.S. often measure sales in “thermal units” or therms. 1 therm = 100,000 Btu Power Power is used to describe energy flow. Power is defined as “the time rate of doing work” and measured in joules/second. SI unit = watt (W) in honor of James Watt inventor of steam engine. 1 watt = 1 joule/second USCS unit = horsepower (hp) where 1 hp = 550 ft-lb/s = 746 W Power vs. Energy Most electrical appliances are rated in terms of their power. But just like when filling the tank of a car at the gas station, you must pay for the total number of gallons pumped, not the rate at which you pumped it. So with electricity, we pay for the total number of joules of electricity consumed, not the power or rate it was delivered. kilowatt-hours (kWh) - U.S. electrical energy is measured in kWh 1 kWh = energy to power TEN 100-watt lightbulbs for an hour. 1 kWh = 1,000J/s x 3,600s = 3.6 x 106 J Electric Power Plants - Rated in terms of their capacity to deliver electric power. Ex) Large coal power plant could be rated at 1,000 MW (megawatts). Sometimes MWe, where the “e” on the W stands for “electric” and signals that the rating is for the output capacity of the plant, and not the energy input. Energy Efficiency = Energy produced x 100 Energy consumed PRACTICE (a) A light bulb has an efficiency rating of 5.2 percent. How much energy is produced for every 1.00 joule of electrical energy? (b) Is the energy produced light or heat energy? PRACTICE QUESTIONS 1. Given that 1 kcal of heat is required to increase the temperature of 1 kg of water by 1°C: • a. How many kcals would be required to heat 100 kg of water by 20°C for a bath? • b. How many joules is this? • c. How many Btus? • d. If your water heater can supply 40 kBtu/h, how long will it take to heat this water?