Introduction to Metabolism
advertisement

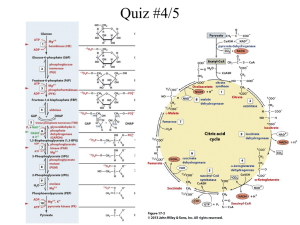
Introduction to Metabolism Metabolic Pathways Laws of Thermodynamics Free Energy Energy Coupling & ATP Enzymes Metabolic Pathways All the chemical reactions in an organism make up its metabolism. There are two types of metabolic pathways: Anabolic and Catabolic The Two Types of Pathways Anabolic: Ex. Dehydration synthesis Photosynthesis Protein synthesis Uses energy Endergonic reactions Catabolic: Ex. Hydrolysis Cellular respiration Releases energy Exergonic reactions Energy and Organisms Remember that energy is the capacity to perform work. Chemical energy is just the potential energy stored in molecules. Catabolic reactions transform potential energy into kinetic energy. Anabolic reactions transform kinetic energy to potential energy. The Laws of Thermodynamics Living systems are subject to the laws of thermodynamics just like cars and furnaces. 1st Law of Thermodynamics (Principle of Conservation of Energy) Energy cannot be created or destroyed. Laws of Thermodynamics (Continued) 2nd Law of Thermodynamics: Every energy transfer or transformation increases the entropy (disorder) of the universe. In living systems, much of the energy involved in transformations is lost as heat. (heat is energy in its most random state) The quantity of energy in the universe is constant but the quality is not. Changes in Living Systems Spontaneous: Occur without outside help (energy) Can be harnessed to perform work Only occur when free energy is available Increase the entropy (disorder) of the universe and increase the stability of the system. Free Energy Portion of a system’s energy that can perform work when the temperature is uniform throughout the system. We can quantify free energy by using the symbols on the next slide. Free Energy Equation G= free energy of a system (a measure of its instability) T=absolute temperature in Kelvins H=total energy of the system S= entropy Relationship is expressed: G=H-TS Free Energy Changes Expressed as delta G (triangle symbol) Systems that spontaneously change to a more stable state must give up energy, give up order, or both. So: Delta G= delta H-T delta S Delta G will have a negative value in these types of changes. Equilibrium and Free Energy Equilibrium is a state of maximum stability. As a reaction proceeds toward equilibrium its free energy decreases. For a reaction at equilibrium delta G is 0.(A reaction at equilibrium performs no work) Exergonic Reactions There is a net release of free energy in an exergonic reaction. Delta G is negative. Magnitude of delta G is the maximum amount of work the reaction can perform. Products store less energy than the reactants. Example Pg.92 Cellular Respiration Endergonic Reactions Stores free energy in molecules Delta G is positive Magnitude of delta G is the quantity of energy required to drive the reaction. Ex. Photosynthesis Products store more energy than the reactants. Metabolic Disequilibrium If cells reached equilibrium they would have no free energy(In other words, they would be dead!) So one of the defining features of living organisms is that they maintain metabolic disequilibrium. They can do this because they are open systems and there is a constant flow of materials in and out of the cell. Energy Coupling and ATP Energy coupling is using exergonic reactions to drive endergonic reactions. ATP is the “energy coupler” for cellular work, like: Mechanical Work-muscle contraction Transport Work- Na-K Pump Chemical Work- protein synthesis (or pushing endergonic reactions) Adenosine Triphosphate Structure: Nitrogenous base adenine bonded to ribose with three phosphate groups attached Reaction: ATP + Water yields ADP + inorganic phosphate Under cellular conditions(pg 94) this reaction yields about –13kcal/mol Characteristics of ATP Bonds between phosphates are unstable because phophates are negatively charged. Release of energy is due to instability-in losing a phosphate it becomes more stable. The terminal phosphate is added (phosphorylation) to another molecule which energizes it. Most cellular work is done by ATP. Enzymes It is important to understand that enzymes are essential to chemical reactions in our bodies. Normally, chemical reactions occur very slowly and would not allow life as we know it to exist. Enzymes make everything possible by speeding up reactions as they lower activation energy. Enzymes (continued) Enzymes are catalytic proteins-they change the rate of reaction without being used up by the reaction. Activation energy is the energy needed to get the reaction to proceed. Your text refers to it as the amount of energy needed to push the reactants over an energy barrier so that the downhill part of the reaction can proceed. Enzymes (continued) Proteins have specific shapes and enzymes are proteins. Remember that how one molecule recognizes another is related to its shape. Therefore, sucrase only recognizes and reacts with sucrose. It will not react with maltose. Enzymes (continued) Enzymes have an active site, a pocket or groove on the surface of the enzyme where the substrate, or substance the enzyme acts on or changes. Once the substrate enters the active site, it makes the enzyme slightly change its shape. The aligns molecules so that the ability of the enzyme to catalyze reactions is enhanced. This is called induced fit. Enzyme Regulation Enzymes are regulated naturally by temperature and pH. They function most effectively in humans within the range of human body temperature. Enzymes in humans generally function best between pH 6-8. An exception would be pepsin which functions best in the stomach at a pH of 2. Enzyme Helpers Cofactors and coenzymes are substances that aid enzymes in their catalytic activity. Some cofactors are minerals like Zinc and Copper. (The minerals in vitamins and mineral supplements) Coenzymes are organic molecules that help enzymes. (some vitamins are cofactors) Enzyme Inhibitors Some substances can prevent the action of enzymes, either totally or temporarily. These are called inhibitors. If they bond covalently to the enzyme, the effect is permanent. If the bonds are weak then the action can be reversed. Competitive and Noncompetitive Inhibitors. Competitive inhibitors mimic the substrate and compete with the substrate for a place in the active site. They can be overcome with more substrate in the environment. Noncompetitive inhibitors do not mimic the substrate but change the shape of the enzyme by binding to it somewhere else making the active site essentially inactive. Metabolic Control The body needs not only speed in chemical reactions but control so tasks are done in order and efficiently. It can regulate the activity of enzymes once they are made or turn genes on and off to make enzymes as needed. Methods of Metabolic Control The allosteric site provides a place for activator and inhibitor molecules to bind and (you guessed it)activate or inhibit the enzyme. Allosterically regulated enzymes often have binding sites where polypeptide chains join. The enzyme becomes stable in its active state if an activator binds to it and stable in its inactive state if an inhibitor binds to it. Control of ATP by enzymes Some catabolic enzymes have allosteric sites that bind inhibitors and activators. If there was an excess of ATP, it would bind to these and act as an inhibitor, slowing catabolism.(catabolism regenerates ATP) If AMP (product of broken down ATP) accumulates it binds to these sites, acting as an activator and increasing the rate of catabolism so that more ATP is regenerated. Feedback Inhibition Feedback inhibition is how ATP is controlled in the example on the last slide. The end product, in that case ATP, slows down its own production so the cell is not wasting resources making substances that it already has enough of…… The next slide shows another example of this process. Cooperativity This type of metabolic control relies on the substrate molecule. It is similar to allosteric regulation and induced fit. When one substrate molecule binds to an enzyme’s active site, it “primes” the enzyme to accept more substrate molecules by making all the subunits fit the substrate. Enzyme Complexes Groups of enzymes involved in a particular metabolic pathway can form complexes that keep them in a particular place. This makes metabolism more efficient because it acts like an assembly line, with the product of one enzyme becoming the substrate for the next one. Some enzyme complexes are bound to particular organelles like those in membranes of the mitochondria that perform cellular respiration. Prokaryotic vs. Eukaryotic Cells While prokaryotes are very successful organisms, the complexity of eukaryotes may be partially explained by the compartmentalization of complex reactions on membranes like those present in chloroplasts and mitochondria Prokaryotes do not have organelles or an endomembrane system like eukaryotes The presence of organelles is presently explained by the Endosymbiosis Theory This states that when smaller cells became part of larger cells by predation, some remained functioning in the host cell (pg.5167)