Blood in a dish: in vitro synthesis of red blood cells

Blood in a dish
-----in vitro synthesis of red blood cells
Presented by :Tian Jing
Co-advisor: Dr.Ma and Dr.Jiang
2012.11.25
Background
Anemia
• 2 billion people worldwide and 10% of the US population, with the highest incidence among the elderly.
• Major surgery and trauma;
• A common toxicity of cancer therapies;
• 16 million red blood cell (RBC) transfusions every year in the United States.
Source:www.hudong.com/wiki/sickle-cell%20anemia
Background
Need for RBC transfusions
• Obtained from donors
• Frequent supply bottlenecks
• Infectious risks ;
• Requires costly screening;
• Donors for rare blood types are scarce.
Source:http://tupian.hudong.com/s/%
Background
• Consequently, numerous efforts are underway to expand erythroid precursors and differentiate them in vitro into mature RBCs.
• Furthermore, erythroid precursors may ultimately serve as a novel cell-based therapy providing a renewable source of RBCs.
Background
The first cell-based therapy
• The first successful blood transfusion: from one dog to another in 1665
• In 1667 , a sheep to man transfusion
• The first microscopic identification of RBCs by Antonie van Leeuwenhoek in 1684.
• The first successful human-to-human blood cell transfusion occurred with the treatment of postpartum hemorrhage using a husband-to-wife transfusion [1]
[1]Diamond, L.K. , McGraw-Hill Book Company(1980) .
Source: http://www.dohenes.com/view.asp?id=574
Background
The first cell-based therapy
• The first functional replacement therapy occurred in
1840 with whole blood transfusion treatment of hemophilia.
• The discovery of blood types by Karl Landsteiner in
1901 and earned him a Nobel Prize for Medicine in
1930 [2].
[2]Diamond, L.K. , McGraw-Hill Book Company(1980) .
Source: http://baike.baidu.com/view/1429067.htm
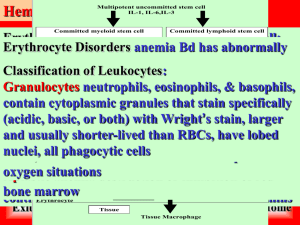
Introduction
Erythropoiesis – the synthesis of RBCs
Hematopoietic stem cells (HSCs); termed burst-forming units erythroid (BFU-E); colony-forming units erythroid (CFU-E); erythroid precursors termed proerythroblasts
(ProE); basophilic erythroblasts (BasoE);polychromatophilic erythroblasts (PolyE) ; orthochromatic erythroblasts (OrthoE); reticulocytes (Retic)
Introduction
In vitro production of RBCs
• This complex process of erythropoiesis, consisting of progressive phases :
• (1) Progenitor expansion;
• (2) Precursor amplification and maturation ;
• (3) Reticulocyte remodeling into terminal RBCs.
Introduction
In vitro production of RBCs: the 2-step erythroid culture system
Twenty years ago, Fibach [3] developed a liquid culture system that included two sequential steps:
• The first step contained glucocorticoids and conditioned media providing cytokines to promote erythroid ‘progenitor’ proliferation ;
• The second step contained EPO alone to promote survival of latestage erythroid progenitor and maturation of erythroid precursors.
dexamethasone (Dex) ; erythro-myeloid progenitors (EMP)
[3] Fibach, E. Haematologia (1991).
Introduction
Improvements of 2-step erythroid culture system
• The first step has been improved by the replacement of conditioned media with several defined cytokines [4] :
• SCF ;
• low concentrations of IL3 ;
• GM-CSF;
• EPO;
• To expand the number of BFU-E and maintain the survival of late-stage erythroid progenitors.
[4]Malik, et al. Blood (1998) .
Introduction
Improvements of 2-step erythroid culture system
• It was also recognized that estradiol, as well as glucocorticoids, can inhibit erythroid maturation and lead to expanded numbers of erythroid ‘progenitors’ in the first phase of erythroid culture [5] .
[9] Migliaccio, G. et al. Blood Cells Mol(2002).
Introduction
Improvements of 2-step erythroid culture system
• The addition of insulin and thyroid hormone to EPO [6] ;
• Molecules antagonistic to the action of glucocorticoids and estrogens [7] ;
• DMSO, ferrous citrate and transferrin [8] ;
• Humanized serum proteins [9] .
[6] Leberbauer, C. et al. . Blood (2005).
[7] Miharada, K. et al. . Nat.Biotechnol (2006)
[8] Maggakis-Keleman, C. et al. Biol. Eng. Comput(2003).
[9] Migliaccio G. et al . Cell Transplant (2010) .
Introduction
Improvements of 2-step erythroid culture system
• The 2-step liquid cultures of human erythroid cells have traditionally generated less than 50% enucleated
RBCs.
• Enucleation rates were dramatically improved by coculture of erythroid precursors on a specific murine bone marrow (MS5) stromal cell line [10] .
• Efficient enucleation has also been facilitated using feeder-free conditions [11] .
• This is an important issue because the production of clinically useful RBCs in vitro will require strategies to avoid exposure of cellular products to nonhuman cells.
[10] Giarratana, M.C. et al. Nat. Biotechnol (2005) .
[11] Miharada, K. et al. Nat. Biotechnol (2006).
Introduction
Improvements of 2-step erythroid culture system
Culture protocol for the efficient production of enucleated red blood cells without feeder cells from hematopoietic stem/progenitor cells.
Passage I ∼ III are the steps to expand erythroid progenitor cells. Passage IV is the step to induce enucleation of progenitor cells [12] .
A MAP, mixture of D-mannitol, adenine, and disodium hydrogen phosphate dodecahydrate.
B nearly 80% of
RBCs were enucleated
[12] Miharada, K. et al. Nat.Biotechnol (2006).
Introduction
Improvements of 2-step erythroid culture system
• Immature, multipotent hematopoietic progenitors have also been expanded in vitro by culture not only with cytokines but also by using human stromal cells transduced with hTERT [13] hTERT: human telomerase catalytic subunit gene-transduced stromal cell
[13] Fujimi, A. et al. Int. J.Hematol (2008)
Nearly 100% of the erythroblasts obtained from third-phase culturing with macrophages were enucleated in the medium both on day 36 and day 38
The recovery rate of
RBC from the day 38 culture from filtration was 80.8 %
1.76 × 10 9 RBC were obtained from 500 CD34+ cells by the four-phase
‘‘stroma-supported macrophage co-culturing system’’ on day 38
Summary
Ultimate goal
• Enucleated RBCs ;
• Oxygen delivery potential similar in vivogenerated RBCs :
• Hemoglobin content,
• Oxygen dissociation characteristics,
• Membrane deformability,
• In vivo lifespan when injected into immunodeficient mice
CD71, transferrin receptor; TER119, a cell surface antigen specific for mature erythroid cells.
Conclusion
The problem of scale
• The RBC products require the ex vivo generation of cell numbers [14];
• The costs associated with ex vivo erythroid cell expansion and differentiation;
• The tumorigenic potential [15] ;
• The establishment of an immortalized human erythroid cell line lacking the genes to produce A, B, and RhD antigens .
[14] Giarratana, M.C., et al. Blood (2011).
[15] H. Hentze,et al. Trends in Biotechnology, (2007).