Therapy(NoTP)
advertisement
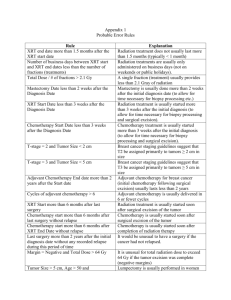
Modalities in Cancer Therapy Folder Title: Therapy(NoTP) TtlTreat Updated: April 13, 2013 Complete The Introduction to Taylor Black Story by TPF Taylor Black 60-Minutes Story Later Why We Do Cancer Therapy Research Read: “In the Elevator at the Princess Margaret Hospital” See tpfondy.mysite.syr.edu Also on course web-site. Therapeutic Modality Options Surgery Radiation Chemotherapy (Including Hormonal Therapy) See Chapter 16: Rational Treatment of Cancer Immunotherapy See Chapter 15: Crowd Control – Tumor Immunology and Immunotherapy Host Response Modification Gene Therapy (and Virotherapy?) “Specific-Targeted” Therapies: Monoclonal antibodies or pharmaceuticals directed to errors in signaling pathways, to signal receptors, or to other key oncoproteins or suppressor proteins. Features of Surgical Intervention • Highly Tumor-specific and Effective • Technically Sophisticated and of Limited Applicability • Access depends on economic status of country* *See Cancer in the Developing World (later) • Useless for Extensive Systemic Disease or for Unrecognized Metastases • Depends on Pathological Identification: – (Must know where the cancer is) • Traumatic, Immunosuppressive, & Dangerous • Can Induce Systemic Spread • Patient Refusal and Opting for Alternative Medicine • Physical Modality – Emergence of Cellular Resistance is not possible Therapies Involving Electromagnetic Radiation: Part 1: X-Ray • External physical intervention with a chemical target (DNA) • Not Especially Tumor-specific; Damage to Normal Tissue Can Limit Utility • Limited Utility Against Extensive, Systemic Disease • Can be Valuable for Debulking Large Local Tumor or Reducing Tumor Size to Permit Surgery • Second Malignancies Can Be Induced • Combination with Radio-sensitizers Can Improve Anti-tumor Specificity • Limited by Tumor Heterogeneity and Selection for Radiation-resistant Variants Radiate Therapies Involving Electromagnetic Radiation: Part 2: Photodynamic Therapy, Hyperthermia, Neutron Capture, Microwave or Radiowave Therapy • External physical intervention with a physical target: – (the cancer cell or cancer mass) • Must know where the cancer mass is – – – – Must be able to direct the physical intervention e.g. Laser-directed microwave Utility against cancers not accessible to surgery May use antibody to bind metal to tumor target for microwave destruction • Damage to normal tissue must be circumvented • Emergence of resistance is unlikely Radiate Possibilities with Ultra-sonic Radiation: Part 1 External physical intervention with a physical target: the size and sonic sensitivity of the cell. Emergence of cellular resistance based on cancer cell biochemistry is not possible. Target is a necessary physical state (enlarged cell size at mitosis) through which the dividing cancer cell must pass, no matter how it got to that state. May not have to know where the cancer cells are. Possibility of Whole-Body Ultra-sound* * Poster by Kenny Shin, Matthew Koslow, and Alison Eimer on whole body sonication of Zebrafish. Should be applicable to metastasizing cells including carcinomas and sarcomas in the circulation or in the lymphatic drainage. Treatment of circulating blood in extra-corporeal shunt or in appendages: (See hand-cooling used by San Francisco 49 er’s). See poster by Ryan Burns, Lindsay Rechan, Alexis Lodico, Ashley Nieves, and Karina Acevedo on sonication of flowing leukemia cells in a glass-coil. Possibilities with Ultra-sonic Radiation: Part 2 Possible to magnify the differences in sonic sensitivity between normal and cancer cells by treatment with cytoskeletal-directed agents or other agents that preferentially affect leukemia cell size. Chemotherapeutic agents produce cell enlargement in cells that are damaged that may still remain viable. Possible to link ultra-sonic therapy with cell cycle-directed chemotherapy. Resistance of red blood cells to low frequency ultra-sound is key to the concept. Tumor-specific or tumor selective anti-neoplastic agents: Chemotherapy Cell-cycle Directed Anti-neoplastic Drugs Cell Cycle Phase Drug Target Go – G1 Taxol Microtubules (stabilize) S-Phase Ara-C (Cytosine arabinoside) DNA synthesis S- G2 VP-16 (Etoposide) Topoisomerase II M Vinca-alkaloids Taxol Microtubule disrupters Microtubule stabilizer Non-cell-cycle specific Alkylating agents: Cis-platinum Cyclophosphamide Nucleophiles (e.g. DNA) Immunotherapy of Cancer • Potentially Highly Tumor-Specific • Can be Effective Against Disseminated Disease Including Unrecognized Micro-metastases • Probably of Limited Value Against Extensive Advanced Disease • Can Involve Severe, Sudden Onset Life-threatening Treatment-limiting Side-Reactions • Limited by Tumor Heterogeneity, Selection for Unresponsive Variants, and Emergence of Immune-Escape ImmunOpt Host-Response Modification in Cancer Management Potentially Less Intrusive than Other More-Aggressive Modalities Treating Host Supporting Cells to Reduce their ability to promote tumor growth (e.g. anti-angiogenesis) Host stromal cell interactions supporting tumor growth: “Respect Thy Neighbor!” Science, Feb. 6, 2004 (BIO 501 Web-Site: Password-protected Link) Host Mod Gene Therapy for Cancer • Potentially Highly Tumor-Specific • Accessibility of Cell Targets Is a Major Obstacle for General Application • May Have Great Value in Combined Modality Approaches • Potentially Dangerous Side-Reactions from Viral Vector Delivery Agents GeneTher “Viro-Therapy” Using Viruses to Treat Cancers (See Scientific American, October 2003, pp 69 to 75 Virotherapy with Transductional Targeting: Adenovirus engineered to bind to an infect only cancer cells Does not infect normal cells Adenovirus multiplies in cancer c ells. Cancer cell burst and disperse virus to infect other cancer cells. Virotherapy with Transcriptional Targeting: Adenovirus engineered to replicate under control of tumor promoter genes. Virus replicates only in cancer cells that have the tumor-specific promoter. Cancer cell bursts and disperse virus particles to infect other cancer cells. Lethal immune-responses in persons sensitized to adenovirus vector. Will show photos of adenovirus virotherapy using Document Camera Virotherapy with Transductional Targeting Scientific American, October 2003 Virotherapy with Transcriptional Targeting. Scientific American Combined Modality Therapies for Cancer Surgery and Radiation Adjuvant Chemotherapy: Surgery and Chemotherapy Radio-sensitizers: Chemotherapy and Radiation Chemotherapy and Host-Response Modification • Induction of Differentiation by Chemotherapeutic Agents • Induction of Apoptosis by Chemotherapeutic Agents Immunotherapy and Gene Therapy Genetically Engineered T-Cells Chemotherapy with Ultra-sonic Disruption? Combined Emergence of Drug Resistance Figure 16.23 The Biology of Cancer (© Garland Science 2007) Table 16.2 The Biology of Cancer (© Garland Science 2007) The picture below is showing an important mechanism involving cancer drug resistance. What is the structure that is shown inserted into the cell membrane? 0 of 90 Physician’s Desk Reference Oncology Reference Guide 203 Oncology Treatment Agents Published in 2003 Costs of Cancer Treatment vs Efficacy Non-small-cell Lung Cancer Treatment with Erbitux (cetuximab) 18 Week course of treatment, $40,000 Average Life Span Increase vs Standard Therapy: 1.2 Months Avastin (bevacizumab) $30,000 to $62,000 per patient per course of treatment. Efficacy marginal Costs of non-curative treatments Off-Label use of approved medications See Science, March 25, 2011, p. 1545-7, David Malakoff Science, Vol 331, March 25, 2011, p. 1547. Average Costs for a single patient. 1st year after diagnosis vs continuing care vs terminal year care Ratio of Mortality to Incidence. If the ratio is large the prospects for survival are bleak. 25 Mar p. 1548 Questions for Society and the Health-Care System And You Do We Treat Everyone? Regardless of Condition, Age, or Life Expectancy? Whether the treatments are clearly beneficial or not? Do we pay for off-label drug treatments (using an agent approved for one cancer to treat another cancer for which efficacy has not been tested) Application of Ultra-sound to Attached Cells: In Situ or Metastatic 1. Cell must detach and round up in order to divide. 2. Attached cells can be made to detach by treatment with cytoskeletal-directed agents. 3. Ultra-sound may be applicable to disseminated attached metastatic cells, not just to leukemia-lymphoma. Modulation of Ultra-sonic Treatment 1. Can vary the sonic frequency (wavelength). 2. Can vary the intensity. 3. Can vary the timing to correspond with cell cycle and cell treatment responses. 4. Opportunity for multiple ultra-sonic treatments repeated at minutes, hours, or days. 5. Host toxicity is likely to be manageable.