Local anaesthetics mgmc
advertisement

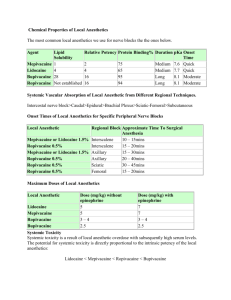
Local anaesthetics Dr. S. Parthasarathy MD., DA., DNB, MD (Acu), Dip. Diab. DCA, Dip. Software statistics PhD (physio) Mahatma Gandhi medical college and research institute , puducherry – India What is it ?? • A drug reversibly blocks the nerve conduction beyond the point of application, if applied in appropriate concentrations . • Other drugs !! • Quinidine, phenergan, TCADs --- no History • Koller is credited with introducing local anesthetics into medical practice when he used cocaine to numb the cornea before operating on the eye. • Isolation of cocaine by Neimann, in 1860 • Procaine was first synthesized in 1904, • In lidocaine 1943 -- but the hierarchy is History ESTERS AMIDES The basic chemical structure- 3 parts: • 1. Lipophilic group- an aromatic group, usually an unsaturated benzene ring. • 2. Intermediate bond- a hydrocarbon connecting chain, either an ester (-CO-) or amide (-HNC-) linkage. The intermediate bond determines the classification of local anesthetic. • 3. Hydrophilic group- a tertiary amine and proton acceptor. COO - ester OR CONH – amide Amides • • • • • • • Bupivacaine Etidocaine Levobupivacaine Lidocaine Mepivacaine Prilocaine Ropivacaine Esters Benzocaine Chloroprocaine Cocaine Procaine Tetracaine Isomerism • Many medications contain chiral molecules which exist as stereoisomers. • Chiral molecules are asymmetrical and the direction of the configuration helps to categorize the isomer. R and S • Polarized light to right – D • Polarized light to left - L Bupi and ropi • Bupivacaine is a long acting amide local anesthetic that can be associated with significant toxicity issues. • S-bupivacaine is almost as potent as the racemic preparation but is less toxic. It takes larger doses of Sbupivacaine to cause cardiac arrest and seizure activity than racemic preparations. • Ropivacaine is a second local anesthetic that is a pure S-ropivacaine. Structure Activity Relationships (Onset, Potency, Duration) pLP=OPD p = pKa = onset L = lipophilicity = potency P = protein binding = duration Frequency dependent block • Local anesthetics are prepared as a water soluble hydrochloride salt and generally have a pH of 5-6. • If the preparation contains epinephrine, the solution must be acidic to create a stable environment. pH of 3-4. • . To enhance clinical onset, carbonated solutions of epinephrine containing local anesthetics have been used instead of HCL solutions. pKa • Because local anesthetics are weak bases, increasing the pH (“alkalinization”) of solution increases the ratio of base to cation. • Henderson-Hasselbalch equation can be used to quantitate the ratio: • pKa(local anesthetic) – pH(solution) = Log ([cation]/[base]) NH3 + HCl = NH4+ + cl- • pKa(local anesthetic) – pH(solution) = Log ([cation]/[base]) • If pH is less , the cationic form is more • If the pH is more the unionized form is more Local anaesthetic Exceptions to pKa • Two notable exceptions are chloroprocaine and benzocaine. • Chloroprocaine has a high pKa and rapid onset. • Benzocaine does not exist in an ionized form and exerts its effects by alternate mechanisms. Lipophilic Nerve cell membrane Pharmacodynamics • Analgesic effect has been reported following intravenous lidocaine administration in many acute and chronic conditions. • Other than Na channels • inhibition of G-protein coupled receptor signaling • Inhibit NGF Differential blockade • Bupivacaine and etidocaine are both potent, long acting local anesthetics. • Bupivacaine exhibits a more potent sensory than motor block. • Etidocaine exhibits an equally effective sensory and motor block. • Ropivacaine, on the other hand, exhibits a potent sensory block similar to bupivacaine but motor blockade appears less intense. most common clinical use of local anesthetics • • • • • Regional anesthesia and analgesia. Topical Infiltration Blocks Neuraxial etc Other actions • Blunt responses to tracheal instrumentation • attenuating increases in intraocular pressure, intracranial pressure, and intra-abdominal pressure during airway instrumentation. Other actions • The primary site of action is the myocardium, where decreases in electrical excitability, conduction rate, and force of contraction occur. • Depressed NMJ • The local anesthetics depress contractions in the intact bowel and in strips of isolated intestine. • also relax vascular and bronchial smooth muscle, Additives • Carbonation of local anesthetics results in a more rapid onset and a more profound degree of conduction blockade, ph higher , More nonionized form, speeder onset , CO2 released diffues inside – acidic- more ionized better action Less tachyphylaxix • Sodabicarb • 1 ml / 20 ml of lignocaine • 0.1 ml /20 ml of bupivacaine Additives • Vasoconstrictors – epinephrine – 1 in 2 lakh – 5 mic/ ml. • Mixtures of local anaesthetics – • EMLA • Ligno + bupi = OK but ?? • A solution containing 50% of the toxic dose of local anesthetic A, and 50% of the toxic dose of local anesthetic B, will have the same implications as 100% of the toxic dose of either local anesthetic alone. Additives • Glucose • The specific gravity of hyperbaric (or ‘heavy’) bupivacaine is 1.026 at 20 ◦C. • The specific gravity of cerebrospinal fluid is 1.005 at 37 ◦C, • Warming of the local anesthetic solutions can also bring about a modified onset time Additives • Hyaluronidase, supplied as a white fluffy powder, is used to facilitate the spread through connective tissues following subcutaneous or intramuscular injection. Additives • • • • Drug Receptor Uses Opioids / mu and kappa Central ,periph Clonidine 2-adrenoceptor Central periphe Ketamine NMDA Central Duration of Action . Local anesthetics are classified as follows: • Short acting: procaine and chloroprocaine • Moderate acting: lidocaine, mepivacaine, prilocaine • Long acting: tetracaine, bupivacaine, etidocaine, ropivacaine, levobupivacaine Pharmacokinetics Metabolism • The metabolism : ester vs. amide. • Ester local anesthetics undergo extensive hydrolysis in the plasma by pseudocholinesterase enzymes (plasma cholinesterase or butyrylcholinesterase). - rapid, resulting in water soluble metabolites which are excreted in the urine. • The ester that is an exception is cocaine. In addition to ester hydrolysis cocaine is partially metabolized in the liver (N-methylation). Metabolism Procaine and benzocaine are metabolized to paminobenzoic acid (PABA), which has been associated with allergic reactions When ester local anesthetics are placed in the CSF, metabolism does not occur until there has been vascular absorption of the local anesthetic. CSF does not contain esterase enzymes. Metabolism • Amide local anesthetics are metabolized primarily by microsomal P-450 enzymes in the liver (Ndealkylation and hydroxylation) and, to a lesser extent, in other tissues. • Most studied lignocaine • Monoethyl glycine xylidide --- xylidine Some drug interactions Side effects • The hydrolysis of all ester-linked local anesthetics leads to the formation of paraaminobenzoic acid (PABA) or a substituted PABA. • True allergic reactions are associated with amino ester-linked local anesthetics, not amino amide-linked one • Tissue Toxicity • Myotoxicity and neurotoxicity Cardiovascular Toxicity • bupivacaine exhibits a much stronger binding affinity to resting and inactivated sodium channels than lidocaine • Bupivacaine dissociates from sodium channels during cardiac diastole much more slowly than lidocaine • Hence bupi cardiotoxicity is more dangerous Methemoglobinemia • The metabolism of prilocaine in the liver results in the formation of O-toluidine, which is responsible for the oxidation of hemoglobin to methemoglobin. • The methemoglobinemia associated with prilocaine is spontaneously reversible or may be treated by IV methylene blue. Toxicity • IV > tracheal > intercostal > caudal > paracervical > epidural > brachial > sciatic > subcutaneous Treatment of Systemic Toxicity from Local Anesthetics • • • • • Prevention aspiration for blood, use of a small test dose of local anesthetic slow injection fractionation of the rest of the dose of local anesthetic Treatment of systemic toxicity is primarily supportive • Injection of local anesthetic should be stopped. • Oxygenation and ventilation should be maintained • If needed intubate and ventilate • Midaz, thio if seizures , ephedrine IVF • IV lipid for bupi • Can we give propofol?? Neural Toxicity of Local Anesthetics • local anesthetic–induced injury to Schwann cells, inhibition of fast axonal transport, disruption of the blood-nerve barrier, decreased neural blood flow disruption of cell membrane integrity • radiculopathy to be approximately 0.03% and of paraplegia to be approximately 0.0008%. Myotoxicity • Toxicity to skeletal muscle is an uncommon side effect of local anesthetic injection. Individual local anaesthetics --Some pearls Cocaine • • • • • Sympathetic stimulation Vasoconstriction Temperature rise Local 10% solution and paste Brompton mixture with heroin for terminally ill Procaine • • • • • Very short acting– no toxicity weak drug Vasodilator Suxa PABA – allergic reaction • No longer in use Cinchocaine (dibucaine) • Dibucaine number • Scoline apnea Lidocaine • is also used in ointment, jelly, viscous, and aerosol preparations for a variety of topical anesthetic procedures. IVRA Anti arrythmic Pain relief - IV inherent potency, rapid onset, moderate duration of action, and topical anesthetic activity Mepivacaine • similar to that of lidocaine • It is ineffective as a topical anesthetic agent. • The metabolism of mepivacaine is greatly prolonged in the fetus and newborn; not employed for obstetric anesthesia Drug doses and toxic doses • Procaine • 7 mg/kg; not to exceed 350-600 mg • Chloroprocaine • Without epinephrine: 11 mg/kg; not to exceed 800 mg total dose With epinephrine: 14 mg/kg; not to exceed 1000 mg • Prilocaine • Body weight <70 kg: 8 mg/kg; not to exceed 500 mg Body weight >70 kg: 600 mg • Ropivacaine • 5 mg/kg; not to exceed 200 mg for minor nerve block • Lidocaine • Without epinephrine: 4.5 mg/kg; not to exceed 300 mg • Lidocaine with epinephrine With epinephrine: 7 mg/kg • Bupivacaine • Without epinephrine: 2.5 mg/kg; not to exceed 175 mg total dose • Bupivacaine with epinephrine • With epinephrine: Not to exceed 225 mg total dose What does a local do ?? 1. 2. Local anesthetic is deposited near a nerve. A portion of the local anesthetic is removed due to tissue binding and circulation. 3. If the local anesthetic is an ester, a portion of the deposited local anesthetic will be removed by local hydrolysis, in addition to tissue binding and circulation. 4. The remaining local anesthetic penetrates the nerve sheath. What does a local do ?? • 5. Local anesthetic penetrates the axon membranes and axoplasm. • This step is dependent on pKa and lipophilicity. • 6. Local anesthetic binds to Na+ channels preventing their opening by inhibiting conformational changes • 7.Local anesthetics may also bind to the channel pore and block the passage of Na+. What does a local do ?? • 8.During incomplete. onset, impulse Partially blockade blocked fibers is are inhibited by repetitive stimulation. The reverse is true during recovery. • 9. The primary route for local anesthetics is the hydrophobic route, within the axon membrane. What does a local do ?? • 10. Clinical onset of blockade is due to the slow diffusion of local anesthetic molecules into the nerve, NOT by binding to ion channels and inhibition of impulse propagation, which occurs at a faster rate. Recovery occurs in reverse. • LA s have no role in RMP. • Individual detailing of local anaesthetics are not done in the hope • That the individual postgraduates will read Thank you all