Malaria
advertisement
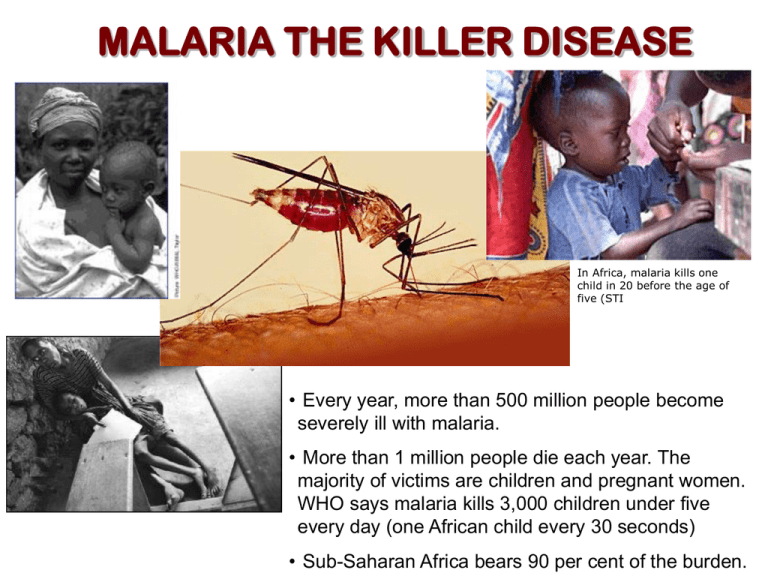
MALARIA THE KILLER DISEASE In Africa, malaria kills one child in 20 before the age of five (STI • Every year, more than 500 million people become severely ill with malaria. • More than 1 million people die each year. The majority of victims are children and pregnant women. WHO says malaria kills 3,000 children under five every day (one African child every 30 seconds) • Sub-Saharan Africa bears 90 per cent of the burden. History of malaria • The periodic fever episodes characteristic for malaria have been described by physician early on in human history • People recognized a connection between malaria and swamps • It was thought that swamps exude a miasma or poison which causes the disease Giovanni Maria Lancisi (1654-1720). He first described a characteristic black pigmentation of the brain and spleen in the victims of malaria. Lancisi linked malaria with poisonous vapours of swamps or stagnant water on the ground. In 1717, in his monograph titled Noxious Emanations of Swamps and Their Cure, he speculates that malaria was due to minute "bugs" or "worms" which entered the blood and revived the old idea that mosquitoes might play a role. History of malaria Dr. Alphonse Laveran, a military doctor in France’s Service de Santé des Armées (Health Service of the Armed Forces). • Based on epidemiological considerations Alphonse Laveran concluded that “Swamp fevers are due to a germ” • In 1880 he discovered parasite life cycle stage (gamonts and gametes) in the blood of patients with fever which were absent in samples from healthy individuals • After the development of methylene blue (1899) it was possible to identify all the malaria species. History of malaria • Camillo Golgi, an Italian neurophysiologist, established that there were at least two forms of the disease, one with tertian periodicity (fever every other day) and one with quartan periodicity (fever every third day). • He prepared high quality micrographs and described the asexual replication of the parasite within the red blood cell • He observed that fever coincided with the rupture and release of merozoites into the blood stream. • He was awarded a Nobel Prize in Medicine for his discoveries in neurophysiology in 1906. History of malaria Ronald Ross was the first to demonstrate that a mosquito could transmit a (bird) malaria parasite. • Patrick Manson (discovered mosquito transmission of Wucheria) proposed same transmission pathway for malaria. • With Manson’s encouragement and advise, Ross finds that the mosquito can get the parasites from human when feeding. • He works with bird malaria in Calcutta and discovers how the parasite is passed from mosquito to the birds (saliva). • He received the Nobel Prize for his work History of malaria • Battista Grassi demonstrates that mosquitoes are also involved in the transmission of human malaria • He identifies the major vectors and life cycles for different Plasmodium species in Italy • Enemies with Ross, he was ignored by the Nobel committee. Nobel Prizes for Malaria Related Research • Ronald Ross, 1902: For his work on malaria, he shows how the parasite enters the organism. He laid the foundation for successful research on this disease and methods of combating it. Ronald Ross demonstrated the oocyst of malarial parasite in the gut wall of a mosquito on August 20, 1897 in Secunderabad, India. • Alphonse Laveran, 1907: "In recognition of his work on the role played by Ronald Ross (1857-1932) protozoa in causing diseases". Laveran was the first to notice parasites in the blood of a patient suffering from malaria on November 6, 1880 at Constantine, Algeria. • Julius Wagner-Jauregg, 1927: "For his discovery of the therapeutic value of malaria inoculation in the treatment of dementia paralytica". Wagner-Jauregg developed methods for treating general paresis (advanced stage of neurosyphilis) by Laveran (1845-1922) inducing fever through deliberate infection of patients with malaria parasites. This method was used in the 1920s and 1930s. In the 1940s, the advent of penicillin and more modern methods of treatment made such "malaria therapy" obsolete. • Paul Hermann Müller, 1948: "For his discovery of the high efficiency of DDT as a contact poison against several arthropods” Jauregg (1857-1949) • Camilo Golgi, 1906: Golgi shared the Nobel Prize with Santiago Ramon Cajal for their studies on the structure of the nervous system. Golgi made significant contributions to malaria research as well Muller(1899-1965) Malaria is caused by plasmodium parasites The parasite spends part of its life cycle inside the red blood cells Humans act as intermediate hosts where sexual and asexual forms of the parasite are found The parasite is transmitted by the bite of the female anopheline mosquito which acts as the definitive host Plasmodium species (Malarial Parasites) • Plasmodium falciparum: malignant tertian malaria. Tropics. Accounts for 50% of all malaria cases. Most pathogenic. • Plasmodium vivax: causes benign tertian malaria. Tropics, subtropics, and some temperate regions. Mostly found in Asia. About 43% of all malaria cases. Some Africans are refractory to infection because the lack the red cell receptor that the parasite use to enter. • Plasmodium malariae: quartan malaria. Tropics. About 7% of malaria cases • Plasmodium ovale: mild tertian malaria. West Africa, occasionally East Africa. Rare. It was used to treat syphilis. HOSTS DEFINITIVE HOST:Sexual stages of the parasite. Anopheline female Mosquito (sexual reproduction). 400 species and one-tenth are potential malaria vectors INTERMEDIATE HOST: Humans (asexual and sexual phases) PARASITE FORMS Merozoite: invades erythrocytes Sporozoite invades mosquito salivary glands and liver cells Ookinete invades mosquito gut epithelial cells Mosquito forms: gametocytes, Oocyst, ookinete, sporozoite Blood forms: merozoites, rings, trophozoite, schizonts Malaria Life Cycle Oocyst Sporozoites Mosquito Salivary Gland Zygote After an indeterminate number of asexual generations, some merozoites become gametocytes Preerythrocytic cycle Gametocyte s IRC ruptures Female anopheline mosquito injects sporozoites which invade liver cells In the liver parasite changes into trophozoite releasing more merozoites Erythrocytic cycle Merozoite transform into trophozoite, ring stage, schizont. Merozoites are released into the blood Plasmodium life cycle in the mosquito Gametocytes develop into gametes: macrogametocyte to macrogamete. Microgametocyte displays exflagellation to form microgametes 10-12 days: sporozoites enter salivary glands 9-11 days: sporozoites exit oocyst in hemolymph 25-50 hours: ookinete transforms into oocyst in midgut epithelium 15-60 min after blood meal: male and female gametes form zygote, which develops into ookinete 24-40 hours: ookinete penetrates and crosses blood meal sac Sporozoite development takes 10-14 days depending on the plasmodium species. Mosquito remains infective for life Anopheles spp. live long enough to feed on human blood repeatedly Infection appears to stimulate mosquito to feed more frequently Exoerythrocytic Cycle Plasmodium sporozoites (green) are deposited under the skin of the vertebrate host through the bite of an infected female Anopheles mosquito. After injection into the skin, the sporozoites move through the dermis until they contact blood vessels (red) and move into the circulatory system, which allows them to travel to the liver. A small proportion of sporozoites can enter the lymphatic system (yellow). Nature Reviews Microbiology (2006): 4-849 The sporozoite After traversing several host cells the sporozoite settles in one and develops and replicates within a welldelimited parasitophorous vacuole. Each invading sporozoite develops and multiplies inside a hepatocyte, forming the schizont, which is made up of thousands of merozoites. In and out. The sporozoite traverse the cytosol of the host cell. Nature Reviews Microbiology (2006): 4-849 Hypnozoites in P. vivax and P.ovale. Dormant forms that seem to be responsible for relapse months or years later. Same section of liver observed by immunofluorescence showing a fluorescent liver schizont but also a smaller fluorescent hypnozoite The sporozoite • 10-15 m long • 2 sporozoite surface proteins contain hepatocyte adhesive domains (CSP circumsporozoite protein and TRAP thrombospondin related anonymous protein) • Both proteins bind to glycosoaminoglycans on the surface of hepatocytes and this binding is important for entry SEM of a sporozoite of Plasmodium cynomolgi. Reaction between the surface antigen (circumsporozoite protein (CSP) and immune serum. The sporozoite would normally have a smooth surface, but the formation of antigen-antibody complexes has created this roughened appearance. The first synthetic malaria vaccine was based upon the CSP repeat sequence in P. Exoerythrocytic Cycle • Schizonts take 5-7 days to develop • formation of >10,000 merozoites • upon rupture of hepatocyte, released merozoites will invade rbc’s The final step involves the release of merozoites (green) into the bloodstream. The signal(s) that trigger the release remain unknown. Plasmodium merozoites are released by the formation of merozoite-filled vesicles (merosomes), which bud off from the infected hepatocytes into the sinusoidal lumen. Nature Reviews Microbiology (2006): 4-849 Merozoites rapidly enter erythrocytes At schizogony, the parasite undergoes multiple rounds of mitosis to generate nuclei that are assembled into the daughter merozoites. At 48 hours, the infected erythrocyte ruptures to release merozoites. Merozoites invade erythrocytes and for this the apical organelles of the merozoite (dense granules, rhoptries and micronemes) must be oriented first. Invasion results in the formation of an intracellular 'ring'-stage that is surrounded by a parasitophorous vacuolar membrane (PVM). After approx. 24–30 hours the trophozoite stage is reached. Rings and trophozoites export parasite proteins into the cytoplasm and membrane of the erythrocyte, and into structures called the Maurer's clefts. PfEMP1, P. falciparum erythrocyte membrane protein 1 The merozoite The merozoite is briefly present in the blood between leaving one red blood cell and entering the next one. Lemon shape, Aprox 1 by 1.6 m micron. In this scanning electron micrograph, a merozoite (the small sphere) is attached to an erythrocyte at the start of invasion.(Image from Bannister, L.H. "Malaria", Topics in Inernational Health,(1998) The WellcomeTrust, CABI Publishing, CAB International) Cowman and Crabb, (2006) Cell 124: 755 The merozoite RBC cell surface molecules act as receptors for merozoite ligands: • P. vivax: Duffy antigen binding protein binds to the duffy antigen • P. falciparum: EBA-175 binds to glycophorin A, unknown ligands bind to glycophorin B and receptor “X” This is TEM which shows the invasion of a red blood cell by a merozoite. The apical complex (at the left side of the merozoite) is attached to the red cell. One of the rhoptries is visible close to the apical complex. Observe dense granules (Image from Aikawa M. "Malaria", Topics in Inernational Health,(1998) The WellcomeTrust, CABI Publishing, CAB International) Plasmodium blood forms: the ring stage • 1st 14-16 hours spent as ring stage, or young trophozoite • little to no Hb degradation • only form seen in blood films of P. falciparum A ring stage of the cup-like form showing the nucleus (n), surrounded by ribosomes and some endoplasmic reticulum. (A Brief Illustrated Guide to the Ultrastructure of Plasmodium falciparum Asexual Blood Stages. L. H. Bannister, J. M. Hopkins, R. E. Fowler, S. Krishnac and G. H. Mitchell, Parasitology Today, 16 (10), pages: 427-433) THE TROPHOZOITE • It is the form found inside the erythrocyte after 10-18 hours post-infection • It interact with the host cell in a very sophisticated way: 1) it takes up the red blood cell hemoglobin and digests it inside a food vacuole. Many of the drugs in use target this compartment; 2) it transports proteins from the parasite to the surface of the erythrocyte for its own benefit. The digestive vacuole of the trophozoite • site of Hb digestion (50-100% digested in < 10 hrs) • several proteases required (plasmepsins and cysteine proteases) THE SCHIZONT • Schizogony: form of asexual reproduction in which multiple mitoses take place, followed by cytokinesis resulting in multiple daughter cells • multiple mitoses produce 20-24 nuclei • once nuclei & organelles replicated cytokinesis occurs • rupture of RBC membrane releases merozoites TEM which shows a schizont of Plasmodium yoelii (rodent malaria parasite). Merozoites surround a residual body (the circular area to the right lower center). Within the apical areas of the merozoites we can see up to two densely-staining rhoptries with finer micronemes. Note the irregular appearance of the red blood cell (RBC) (Image from Ellis DS, Malaria", Topics in Inernational Health,(1998) The WellcomeTrust, CABI Publishing, CAB International) Schizont from Plasmodium falciparum Gamogony 27, 28: Mature macrogametocytes (female); 29, 30: Mature microgametocytes (male). • Formation of gametocytes • trigger for this is unknown, in in vitro culture it is stress related. • 9-10 day development for P. falciparum • 36 hours development for P. vivax • in P. falciparum have characteristic banana shape Development in the mosquito • • • • Upon ingestion with a blood meal, both the micro and macrogametocyte rapidly mature Macrogamete is released from ruptured rbc Microgametocyte rapidly undergoes multiple nuclear divisions to form 8 gametes Exflagellation THE OOKINETE • Fertilization and fusion of nuclei (diploid zygote) • Transforms to a motile ookinete which migrates to outer midgut wall and encysts A mature ookinete. A number of organelles are shown. The nucleus can be seen at the lower end of the organism. There are abundant ribosomes in the area above the nucleus and endoplasmic reditulum can also be seen. The zygote is surrounded by a three-layered pellicle. The apical complex at the upper end includes numerous rhoptries and micronemes (dark spots). Image from Sinden RE. "Malaria", Topics in Inernational Health,(1998) The WellcomeTrust, CABI Publishing, CAB International EM of P. gallinaceum ookinetes. An ookinete (o) penetrating the perithrophic membrane (PT) 34 h after a blood meal.Inset a: the ookinete is surrounded by fine granular material. Inset b: The ookinete pellicle consists of the ookinete plasma membrane (PM), two inner membranes (IM-1 and IM-2) and a dark fuzzy layer (DL). (Toril et al, J. Protozool. 39(4) (1992), pp.449) Development in the mosquito • Encysted ookinete transforms into oocycst • 10-14 days of development • reductional nuclear division, haploid again • multiplication to form 1000’s of sporozoites THE OOCYST SEM which shows two oocysts on the outer wall of the midgut of a mosquito. These contain developing P. gallinacium sporozoites. Image from Guggehheim R."Malaria", Topics in Inernational Health,(1998) The WellcomeTrust, CABI Publishing, CAB International) Anopheles gambiae, the deadliest malaria vector (top), and blue-colored Plasmodium oocysts, appearing from the mosquito’s gut. (MOSQUITO ENGINEERING:Building a Disease-Fighting Mosquito. Martin Enserink/Science 2000 290: 440-441. (in News Focus) The sporozoite invades the mosquito salivary glands Sporozoites which are released from the oocysts come in contact, and invade only the salivary glands. The mechanism of invasion has been studied but still is not well characterized which are receptors for sporozoite invasion. The figure shows sporozoites entering the salivary glands and assembling in bundles in the secretory cavity . A small number of parasites enter the secretory duct. BL: basal lamina; DW: duct wall; N: nucleus; n: nucleolus; PM: plasma membrane; SC: secretory cavity. Current opinion in microbiology, 2009, 12:394 SEM of sporozoites from an opened oocyst which has developed on the outer surface of a mosquito Anopheles stephensi midgut. The parasite is Plasmodium falciparum. (Image from Guggenheim R. "Malaria", Topics in International Health,(1998) The WellcomeTrust, CABI Publishing) Chemotherapy of Malaria Spectrum of activity of antimalarial drugs Chloroquine Pyrimethamine Sulfadoxine Quinine Tetracycline Primaquine Artemisinin Sporozoite Liver stage (exoerythrocytic) - -+ + - RBC stages RBC stages Asexual sexual (trophozoites) (gametocytes) + + -+ + -+ + + + - Other drugs: Clindamycin, azythromycin, atovaquone, Fluoroquinolones, others, combinations DIAGNOSIS Malaria should be considered a potential medical emergency Delay in diagnosis and treatment is a leading cause of death Clinical diagnosis: patient’s symptoms and physical examination. The first symptoms (fever, chills, sweats, headaches, muscle pains, nausea and vomiting) are not specific. In severe malaria, clinical findings are more striking (confusion, coma, neurologic focal signs, severe anemia, respiratory difficulties. Microscopic diagnosis: Blood smear stained with Giemsa Antigen detection Molecular diagnosis: PCR Serology: detection of antibodies against parasites EPIDEMIOLOGY Important factors: Female Anopheles mosquito Humans Malaria parasites In rare cases malaria parasites can be transmitted through blood transfusion, organ transplantation or shared needles. Animal reservoirs have no important role Climate can influence all three components of the life cycle: Rainfall Ambient temperature: transmission is greater in warmer areas Human behavior EPIDEMIOLOGY • Anopheles mosquitos: species of anopheles present in the area influence the intensity of transmission. • The females that prefer to feed on humans indoors will be more effective vectors. • Insecticide resistance is an important factor • Biological characteristics influence a person’s malaria risk • Two genetic factors are epidemiologically important: 1) persons who have the sickle cell trait heterozygotes for the abnormal hemoglobin S are resistant; 2) Persons who are negative for the Duffy blood group have rbc resistant to infection by P. vivax. • Pregnancy: women who have developed protective immunity against P. falciparum tend to lose this protection if pregnant • Behavioral factors: • housing, use of bed nets, financial situation, standing water, agricultural work, domestic animals, war migrations MALARIA and PREGNANCY • In high transmission areas, women have gained a level of immunity to malaria that wanes during pregnancy. In these cases, malaria is more likely to result in severe maternal anemia and delivery of lowweight infants • In low transmission areas, women have developed no immunity to malaria. In these cases malaria infections is more likely to result in severe malaria disease, anemia, premature delivery and fetal loss. • As many as 200,000 fetal and infant deaths and 2,500 maternal deaths yearly attributed to increased malaria susceptibility during pregnancy. • Parasitized red cells adhere and accumulate within the placenta. Pregnant woman having a blood smear taken at an antenatal clinic at the Maela Camp in Thailand near the Myanmar border. Pregnant women are at increased risk of malaria. Image contributed by the Shoklo Malaria Research Unit, Mae Sot, Thailand. Each year, approximately 50 million women living in malaria-endemic countries throughout the world become pregnant, of whom over half live in tropical areas of Africa with intense transmission of Plasmodium falciparum. MALARIA AN HIV Worldwide Distribution of Malaria Malaria and HIV overlap in Sub Saharan Africa, Southeast Asia and South America. In 2003 HIV/AIDS caused the deaths of approx. 2.9 M people of whom 2.4 M lived in Africa Worldwide Distribution of HIV, End of 2004 Malaria and HIV interaction studies Summary of current status • HIV-associated immunosuppression contributes to more and worse malaria and it’s consequences in adults, pregnant women, and children. • Malaria contributes to stimulus of HIV replication and possibly(?) to its consequences: disease progression, transmission in adults, and mother to child transmission. • Co-infection with Malaria and HIV in pregnant women contributes to anemia, low birth weight, and their risk for poor infant survival. • Malarial anemia in children too frequently requires blood transfusion and may still lead to HIV transmission A clinician at the HIV clinic of the Provincial General Hospital in Kisumu, Kenya, examines a child hospitalized with fever possibly caused by malaria. (Courtesy Kenya Ministry of Health.) MALARIA CONTROL • Diagnosis and treatment • Prevention of infection through vector control (use of insecticide-treated mosquito nets (shown to reduce all-cause child mortality by 20%-25%) • Prevention of disease by administration of antimalarial drugs to particularly vulnerable population groups such as pregnant women Ali Khamis Abbas, on behalf of the Zanzibar Malaria Control Program, presents a longlasting insecticide-treated net to Consolata John, Chairperson of the Zanzibar Association of People with HIV/ AIDS Living Positively. (Courtesy Patrick Kachur) The nets from all houses in a Tanzanian village are given their annual retreatment free of charge by a nurse from the health centre. Photograph taken by T.J. Wilkes. Malaria control Mosquito Control and ACT (Artemisinin-based combined therapies) are both likely contributors to the Reduction of Malaria in KwaZulu–Natal ROLL BACK MALARIA To provide a coordinated international approach to fighting malaria the Roll Back Malaria (RBM) Global Partnership was launched in 1998 by the World Health Organization, UNICEF, UNDP and the World Bank. RBM’s goal is to halve the burden of malaria by 2010. PRESIDENTIAL MALARIA INITIATIVE • The President’s Malaria Initiative (PMI) represents an historic fiveyear expansion of U.S. Government (USG) resources to fight malaria in the region most affected by the disease. • The President committed an additional $1.2 billion in malaria funding to this Initiative with the goal of reducing malaria-related deaths by 50 percent in 15 focus countries. • This will be achieved by expanding coverage of highly effective malaria prevention and treatment measures to 85 percent of the most vulnerable populations – children under 5 years of age and pregnant women. • This package of high-impact interventions includes insecticide-treated mosquito nets (ITNs), indoor residual spraying (IRS) with insecticides, intermittent preventive treatment for pregnant women (IPTp), and artemisinin-based combination therapy (ACT).








